| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|
| 体内研究 (In Vivo) |
在这种慢性小鼠哮喘模型中,L-肉碱 ((R)-Carnitine)(125、250 mg/kg;腹腔注射)具有较低的尿液 LTE4 排泄和更显着的支气管扩张作用 [2]。
|
|---|---|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Absolute bioavailability is 15% (tablets or solution). Time to maximum plasma concentration was found to be 3.3 hours. Following a single intravenous dose, 73.1 +/- 16% of the dose was excreted in the urine during the 0-24 hour interval. Post administration of oral carnitine supplements, in addition to a high carnitine diet, 58-65% of the administered radioactive dose was recovered from urine and feces in 5-11 days. The steady state volume of distribution (Vss) of an intravenously administered dose, above endogenous baseline levels, was calculated to be 29.0 +/- 7.1L. However this value is predicted to be an underestimate of the true Vss. Total body clearance was found to be a mean of 4L/h. L-Carnitine is a naturally occurring compound that facilitates the transport of fatty acids into mitochondria for beta-oxidation. ... In humans, the endogenous carnitine pool, which comprises free L-carnitine and a range of short-, medium- and long-chain esters, is maintained by absorption of L-carnitine from dietary sources, biosynthesis within the body and extensive renal tubular reabsorption from glomerular filtrate. In addition, carrier-mediated transport ensures high tissue-to-plasma concentration ratios in tissues that depend critically on fatty acid oxidation. The absorption of L-carnitine after oral administration occurs partly via carrier-mediated transport and partly by passive diffusion. After oral doses of 1-6 g, the absolute bioavailability is 5-18%. In contrast, the bioavailability of dietary L-carnitine may be as high as 75%. Therefore, pharmacological or supplemental doses of L-carnitine are absorbed less efficiently than the relatively smaller amounts present within a normal diet. L-Carnitine and its short-chain esters do not bind to plasma proteins and, although blood cells contain L-carnitine, the rate of distribution between erythrocytes and plasma is extremely slow in whole blood. After iv administration, the initial distribution volume of L-carnitine is typically about 0.2-0.3 L/kg, which corresponds to extracellular fluid volume. There are at least three distinct pharmacokinetic compartments for L-carnitine, with the slowest equilibrating pool comprising skeletal and cardiac muscle. L-Carnitine is eliminated from the body mainly via urinary excretion. Under baseline conditions, the renal clearance of L-carnitine (1-3 mL/min) is substantially less than glomerular filtration rate (GFR), indicating extensive (98-99%) tubular reabsorption. The threshold concentration for tubular reabsorption (above which the fractional reabsorption begins to decline) is about 40-60 umol/L, which is similar to the endogenous plasma L-carnitine level. Therefore, the renal clearance of L-carnitine increases after exogenous administration, approaching GFR after high iv doses. ... In mammals, the carnitine pool consists of nonesterified L-carnitine and many acylcarnitine esters. Of these esters, acetyl-L-carnitine is quantitatively and functionally the most significant. Carnitine homeostasis is maintained by absorption from diet, a modest rate of synthesis, and efficient renal reabsorption. Dietary L-carnitine is absorbed by active and passive transfer across enterocyte membranes. Bioavailability of dietary L-carnitine is 54-87% and is dependent on the amount of L-carnitine in the meal. Absorption of L-carnitine dietary supplements (0.5-6 g) is primarily passive; bioavailability is 14-18% of dose. Unabsorbed L-carnitine is mostly degraded by microorganisms in the large intestine. Circulating L-carnitine is distributed to two kinetically defined compartments: one large and slow-turnover (presumably muscle), and another relatively small and rapid-turnover (presumably liver, kidney, and other tissues). At normal dietary L-carnitine intake, whole-body turnover time in humans is 38-119 hr. In vitro experiments suggest that acetyl-L-carnitine is partially hydrolyzed in enterocytes during absorption. In vivo, circulating acetyl-L-carnitine concentration was increased 43% after oral acetyl-L-carnitine supplements of 2 g/day, indicating that acetyl-L-carnitine is absorbed at least partially without hydrolysis. After single-dose intravenous administration (0.5 g), acetyl-L-carnitine is rapidly, but not completely hydrolyzed, and acetyl-L-carnitine and L-carnitine concentrations return to baseline within 12 h. At normal circulating l-carnitine concentrations, renal l-carnitine reabsorption is highly efficient (90-99% of filtered load; clearance, 1-3 mL/min), but displays saturation kinetics. Thus, as circulating L-carnitine concentration increases (as after high-dose intravenous or oral administration of L-carnitine), efficiency of reabsorption decreases and clearance increases, resulting in rapid decline of circulating L-carnitine concentration to baseline. Elimination kinetics for acetyl-L-carnitine are similar to those for L-carnitine. There is evidence for renal tubular secretion of both L-carnitine and acetyl-L-carnitine. ... The pharmacokinetics of L-carnitine and its metabolites were investigated in 7 healthy subjects following the oral administration of 0, 0.5, 1, and 2 g 3 times a day for 7 days. Mean plasma concentrations of L-carnitine across an 8-hour dose interval increased significantly (P < 0.05) from a baseline of 54.2 +/- 9.3 uM to 80.5 +/- 12.5 uM following the 0.5-g dose; there was no further increase at higher doses. There was a significant increase (P <0.001) in the renal clearance of L-carnitine indicating saturation of tubular reabsorption. Trimethylamine plasma levels increased proportionately with L-carnitine dose, but there was no change in renal clearance. A significant increase in the plasma concentrations of trimethylamine-N-oxide from baseline was evident only for the 2-g dose of L-carnitine (from 34.5 +/- 2.0 to 149 +/- 145 uM), and its renal clearance decreased with increasing dose (P <0.05). There was no evidence for nonlinearity in the metabolism of trimethylamine to trimethylamine-N-oxide. In conclusion, the pharmacokinetics of oral L-carnitine display nonlinearity above a dose of 0.5 g 3 times a day. Evidence indicates L-carnitine is absorbed in the intestine by a combination of active transport and passive diffusion. Reports of bioavailability following an oral dose have varied substantially, with estimates as low as 16 to 18% and as high as 54 to 87% ... The mucosal absorption of carnitine appears to be saturated at about a 2-g dose. Max blood concn is reached approx 3.5 hr after an oral dose and slowly decr, with a half-life of about 15 hr. Elimination of carnitine occurs primarily through the kidneys. The heart, skeletal muscle, liver, kidneys, and epididymis have specific transport systems for carnitine that concentrate carnitine within these tissues. Despite evidence indicating incr levels of free carnitine and carnitine metabolites in the blood and urine following an oral dose, no significant change in RBC carnitine levels was noted in healthy subjects, suggesting either a slow repletion of tissue stores of carnitine following an oral dose or a low capability to transport carnitine into tissues under normal conditions. For more Absorption, Distribution and Excretion (Complete) data for L-CARNITINE (11 total), please visit the HSDB record page. Metabolism / Metabolites After oral administration L-carnitine which is unabsorbed is metabolized in the gastrointestinal tract by bacterial microflora. Major metabolites include trimethylamine N-oxide and [3H]-gamma-butyrobetaine. In mammals, L-carnitine is synthesized from epsilon-N-trimethyllysine, which is derived from post-translationally methylated lysine residues in proteins, and protein turnover. In normal humans, the rate of synth is est to be ca 1.2 umol/kg/day. The rate of L-carnitine biosynth is regulated by the avail of epsilon-N-trimethyllysine. Thus, conditions that incr protein methylation and/or protein turnover may incr the rate of L-carnitine biosynth. Synthesis of carnitine begins with methylation of the amino acid L-lysine by S-adenosylmethionine (SAMe). Magnesium, vitamin C, iron, vitamins B3 and B6, and alpha-ketoglutarate - along with the cofactors responsible for creating SAMe (methionine, folic acid, vitamin B12, and betaine) - are all required for endogenous carnitine synthesis. Unabsorbed L-carnitine is degraded by micro-organisms in the large intestine. Major metabolites identified are trimethylamine oxide in urine and gamma-butyrobetaine in feces. Carnitine plays an indispensable role in fatty acid oxidation. Previous studies revealed that fetal carnitine is derived from the mother via transplacental transfer. Recent studies demonstrated the presence and importance of an active fatty acid oxidation system in the human placenta and in the human fetus. In view of these findings ... carnitine metabolism /was studied/ in the fetal-placental unit by measuring carnitine metabolites, intermediary metabolites of carnitine biosynthesis, as well as the activity of carnitine biosynthesis enzymes in human term placenta, cord blood and selected embryonic and fetal tissues (5-20 weeks of development). Placenta contained low but detectable activity of gamma-butyrobetaine dioxygenase. This enzyme, which was considered to be expressed only in kidney, liver and brain, catalyzes the last step in the carnitine biosynthesis pathway. In addition, ... human fetal kidney, liver and spinal cord already have the capacity to synthesize carnitine. The ability of the placenta and fetus to synthesize carnitine suggests that in circumstances when maternal carnitine supply is limited, carnitine biosynthesis by the fetal-placental unit may supply sufficient carnitine for placental and fetal metabolism. /Carnitine/ For more Metabolism/Metabolites (Complete) data for L-CARNITINE (7 total), please visit the HSDB record page. After oral administration L-carnitine which is unabsorbed is metabolized in the gastrointestinal tract by bacterial microflora. Major metabolites include trimethylamine N-oxide and [3H]-gamma-butyrobetaine. Route of Elimination: Following a single intravenous dose, 73.1 +/- 16% of the dose was excreted in the urine during the 0-24 hour interval. Post administration of oral carnitine supplements, in addition to a high carnitine diet, 58-65% of the administered radioactive dose was recovered from urine and feces in 5-11 days. Half Life: 17.4 hours (elimination) following a single intravenous dose. Biological Half-Life 17.4 hours (elimination) following a single intravenous dose. Distribution: 0.585 hours; Elimination: 17.4 hours ... Half-life /in blood/ ca 15 hr ... |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
Levocarnitine can be synthesised within the body from the amino acids lysine or methionine. Vitamin C (ascorbic acid) is essential to the synthesis of carnitine. Levocarnitine is a carrier molecule in the transport of long chain fatty acids across the inner mitochondrial membrane. It also exports acyl groups from subcellular organelles and from cells to urine before they accumulate to toxic concentrations. Only the L isomer of carnitine (sometimes called vitamin BT) affects lipid metabolism. Levocarnitine is handled by several proteins in different pathways including carnitine transporters, carnitine translocases, carnitine acetyltransferases and carnitine palmitoyltransferases. Protein Binding None Toxicity Data LD50 > 8g/kg (mouse, oral). Interactions ... The present study aimed to investigate whether an increase in whole body carnitine retention can be achieved through L-carnitine feeding in conjunction with a dietary-induced elevation in circulating insulin. On two randomized visits (study A), eight men ingested 3 g/day L-carnitine followed by 4 x 500-mL solutions, each containing flavored water (Con) or 94 g simple sugars (glucose syrup; CHO). In addition, 14 men ingested 3 g/day L-carnitine followed by 2 x 500 mL of either Con or CHO for 2 wk (study B). Carbohydrate ingestion in study A resulted in a fourfold greater serum insulin area under the curve when compared with Con (P < 0.001) and in a lower plasma TC concentration throughout the CHO visit (P < 0.05). Twenty-four-hour urinary TC excretion in the CHO visit was lower than in the Con visit in study A (155.0 +/- 10.7 vs. 212.1 +/- 17.2 mg; P < 0.05). In study B, daily urinary TC excretion increased after 3 days (65.9 +/- 18.0 to 281.0 +/- 35.0 mg; P < 0.001) and remained elevated throughout the Con trial. During the CHO trial, daily urinary TC excretion increased from a similar basal value of 53.8 +/- 9.2 to 166.8 +/- 17.3 mg after 3 days (P < 0.01), which was less than during the Con trial (P < 0.01), and it remained lower over the course of the study (P < 0.001). The difference in plasma TC concentration in study A and 24-h urinary TC excretion in both studies suggests that insulin augmented the retention of carnitine in the CHO trials. ... The effect of the anti-cancer drug carboplatin on plasma concentrations and urinary excretion of L-carnitine (LC) and its main ester, acetyl-L-carnitine (ALC), in cancer patients /was examined/. ... Treatment with carboplatin was associated with a marked urinary loss of LC and ALC, most likely due to inhibition of carnitine reabsorption in the kidney. ... The rats were divided into four groups: group 1, control (0.9% NaCl); group 2, doxorubicin (DOX) injection (7.5 mg/kg, i.v.); group 3, DOX plus low dose (40 mg/kg) L-carnitine; and group 4, DOX plus high dose (200 mg/kg) L-carnitine. L-carnitine was administered 1 h before doxorubicin injection and daily thereafter for 15 days. ... Rats in group 2 were associated with hypoalbuminemia, hyperlipidemia, high urinary excretion of protein and elevated plasma creatinine and urea nitrogen. The glomerular filtration rate (GFR) and effective renal plasma flow (ERPF) decreased with increased renal vascular resistance (RVR). Kidney catalase (CAT) activity was decreased. In group 3 and 4, plasma triglyceride and cholesterol declined. L-carnitine improved renal functions by elevated GFR and ERPF and decreased plasma creatinine and urea nitrogen. The kidney CAT activity were increased significantly compared with group 2. From histopathological results, group 2 rats were found to have glomerular capillary dilation and tubular dilation. The lesions were less in group 3 and 4 rats... ... Recent literature documents no cases of allergic reactions or serious side effects associated with the administration of carnitine when given patients with acute ingestions of valproic acid. Other findings suggest that carnitine increases the survival rate of patients who develop valproic-acid-induced hepatotoxicity. Early intervention with iv rather than enteral L-carnitine was associated with the greatest hepatic survival. Isolated pediatric case reports show that carnitine administration may reverse toxic metabolic pathways but may not hasten clinical improvement. .../Carnitine/ For more Interactions (Complete) data for L-CARNITINE (17 total), please visit the HSDB record page. Non-Human Toxicity Values LD50 Rat iv 5.4 g/kg LD50 Mouse oral 19.2 g/kg |
| 参考文献 |
[1]. Agarwal A, et al. Role of L-carnitine in female infertility. eprod Biol Endocrinol. 2018 Jan 26;16(1):5.
[2]. Ferreira GC, et al. L-Carnitine and Acetyl-L-carnitine Roles and Neuroprotection in Developing Brain. Neurochem Res. 2017 Jun;42(6):1661-1675. [3]. Uzuner N, et al. The role of L-carnitine in treatment of a murine model of asthma. Acta Med Okayama. 2002 Dec;56(6):295-301. |
| 其他信息 |
Therapeutic Uses
Levocarnitine is indicated for treatment of primary systemic carnitine deficiency, a genetic impairment of normal biosynthesis or utilization of levocarnitine from dietary sources, or for the treatment of secondary carnitine deficiency resulting from an inborn error of metabolism. /Included in US product labeling/ Parenteral levocarnitine is indicated for the prevention and treatment of carnitine deficiency in patients with end-stage renal disease supported on hemodialysis. /Included in US product labeling/ Levocarnitine oral solution is used for the prevention and treatment of carnitine deficiency secondary to valproic acid toxicity. /NOT included in US product labeling/ L-Carnitine, acetyl-L-carnitine, and/or propionyl-L-carnitine may be used for replacement therapy to restore normal carnitine concn and/or a normal nonesterified-to-esterified carnitine ratio ... For primary and some secondary carnitine deficiencies ... L-carnitine is used for replacement therapy. For more Therapeutic Uses (Complete) data for L-CARNITINE (29 total), please visit the HSDB record page. Drug Warnings Various mild gastrointestinal complaints have been reported during the long-term administration of oral L- or D,L-carnitine; these include transient nausea and vomiting, abdominal cramps, and diarrhea. Mild myasthenia has been described only in uremic patients receiving D,L-carnitine. Gastrointestinal adverse reactions with carnitor (levocarnitine) Oral Solution or carnitor SF (levocarnitine) Sugar-Free Oral Solution dissolved in liquids might be avoided by a slow consumption of the solution or by a greater dilution. Decreasing the dosage often diminishes or eliminates drug-related patient body odor or gastrointestinal symptoms when present. Tolerance should be monitored very closely during the first week of administration, and after any dosage increases. Seizures have been reported to occur in patients with or without pre-existing seizure activity receiving either oral or intravenous levocarnitine. In patients with pre-existing seizure activity, an increase in seizure frequency and/or severity has been reported. ... Oral L-carnitine at a dose of 1 g daily was administered for twelve days to six patients with end-stage renal disease undergoing hemodialysis thrice weekly. Pre-dialysis plasma concentrations of L-carnitine (mean +/- SD) increased significantly (P < 0.05) from day 1 (baseline; 32.4 +/- 6.1 uM) to day 8 (66.1 +/- 13.8 uM) remaining constant thereafter. Although plasma levels of trimethylamine remained unaltered, the pre-dialysis plasma concentrations of trimethylamine-N-oxide increased significantly (P < 0.05) from day 1 (289.1 +/- 236.1 microM) to day 12 (529.0 +/- 237.9 uM). The hemodialysis clearances for L-carnitine, trimethylamine and trimethylamine-N-oxide were 14.3 +/- 8.2, 14.1 +/- 10.6 and 12.4 +/- 5.4 L/h, respectively, indicating their efficient removal by dialysis. Oral administration of L-carnitine at a dose of 1 g daily increases plasma concentrations of this substance to physiological levels in patients with end-stage renal disease who are undergoing hemodialysis. However, concerns about the possible deleterious consequences of such a dosage regimen still remain given that plasma concentrations of trimethylamine-N-oxide were continually rising and approximately doubled in a two-week period. ... Patients with primary carnitine deficiency display alterations in the renal handling of L-carnitine and/or the transport of the compound into muscle tissue. Similarly, many forms of secondary carnitine deficiency, including some drug-induced disorders, arise from impaired renal tubular reabsorption. Patients with end-stage renal disease undergoing dialysis can develop a secondary carnitine deficiency due to the unrestricted loss of L-carnitine through the dialyser ... The aim of our work was to test the influence of L-carnitine supplementation on secondary hyperparathyroidism and bone metabolism in hemodialyzed patients in a randomized study. Eighty-three chronically hemodialyzed patients were observed; 44 were supplemented with L-carnitine (15 mg/kg iv after each hemodialysis for 6 months), while 39 took placebo. Levels of free carnitine (CAR), calcium (Ca), inorganic phosphate (P), Ca x P product, parathormone (PTH), bone-specific alkaline phosphatase (b-ALP), osteocalcin (OC), and osteoprotegerin (OPG) were monitored. In comparison with pretreatment values, changes of some selected parameters occurred in the supplemented patients after 6 months (data are expressed as medians; NS, nonsignificant change): PTH, 186.0 vs. 135.5 ng/L (NS); b-ALP, 13.9 vs. 13.2 ug/L (P < 0.05); OC, 78.3 vs. 68.8 ug/L (NS); OPG, 144.0 vs. 182.0 ng/L (P < 0.05). In the controls, there were the following changes: PTH, 148.0 vs. 207.0 ng/L (NS); b-ALP, 15.2 vs. 13.2 ug/L (P < 0.05); OC, 62.7 vs. 79.8 ug/L (P < 0.05); OPG, 140.0 vs. 164.0 ng/L (NS). A significant correlation was found between CAR and OPG changes (r = 0.51, P < 0.001) in the supplemented patients. The supplementation led to a significant increase of serum OPG concentration. Nevertheless, ...only nonsignificant tendencies to correction of secondary hyperparathyroidism and reduction of bone turnover in hemodialyzed patients supplemented with L-carnitine /were observed/ in contrast to controls. At this point, the use of L-carnitine does not seem to be justified. For more Drug Warnings (Complete) data for L-CARNITINE (9 total), please visit the HSDB record page. Pharmacodynamics Levocarnitine is a carrier molecule in the transport of long chain fatty acids across the inner mitochondrial membrane. It also exports acyl groups from subcellular organelles and from cells to urine before they accumulate to toxic concentrations. Lack of carnitine can lead to liver, heart, and muscle problems. Carnitine deficiency is defined biochemically as abnormally low plasma concentrations of free carnitine, less than 20 µmol/L at one week post term and may be associated with low tissue and/or urine concentrations. Further, this condition may be associated with a plasma concentration ratio of acylcarnitine/levocarnitine greater than 0.4 or abnormally elevated concentrations of acylcarnitine in the urine. Only the L isomer of carnitine (sometimes called vitamin BT) affects lipid metabolism. The "vitamin BT" form actually contains D,L-carnitine, which competitively inhibits levocarnitine and can cause deficiency. Levocarnitine can be used therapeutically to stimulate gastric and pancreatic secretions and in the treatment of hyperlipoproteinemias. |
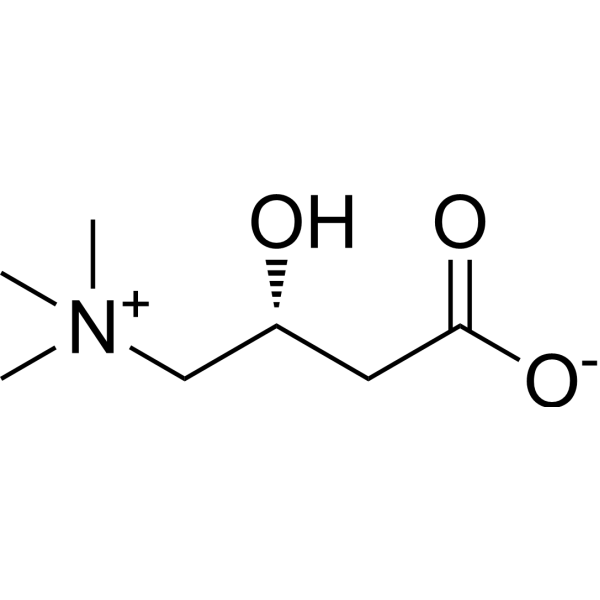
| 分子式 |
C7H15NO3
|
|---|---|
| 分子量 |
161.201
|
| 精确质量 |
161.105
|
| CAS号 |
541-15-1
|
| PubChem CID |
10917
|
| 外观&性状 |
White, crystalline, hygroscopic powder
|
| 熔点 |
197-212 °C(lit.)
|
| 折射率 |
-32 ° (C=1, H2O)
|
| LogP |
-4.52
|
| tPSA |
60.36
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
3
|
| 可旋转键数目(RBC) |
3
|
| 重原子数目 |
11
|
| 分子复杂度/Complexity |
134
|
| 定义原子立体中心数目 |
1
|
| SMILES |
O([H])[C@]([H])(C([H])([H])C(=O)[O-])C([H])([H])[N+](C([H])([H])[H])(C([H])([H])[H])C([H])([H])[H]
|
| InChi Key |
PHIQHXFUZVPYII-ZCFIWIBFSA-N
|
| InChi Code |
InChI=1S/C7H15NO3/c1-8(2,3)5-6(9)4-7(10)11/h6,9H,4-5H2,1-3H3/t6-/m1/s1
|
| 化学名 |
(3R)-3-hydroxy-4-(trimethylazaniumyl)butanoate
|
| 别名 |
L-Cartin; Carnitor; Levocarnitine
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中(例如氮气保护),避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
H2O : ≥ 50 mg/mL (~310.17 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 100 mg/mL (620.35 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶。
请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 6.2035 mL | 31.0174 mL | 62.0347 mL | |
| 5 mM | 1.2407 mL | 6.2035 mL | 12.4069 mL | |
| 10 mM | 0.6203 mL | 3.1017 mL | 6.2035 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Trimethylamine N-oxide Effects of a Pomegranate Supplement Simultaneously With Carnitine (TESSA)
CTID: NCT06518343
Phase: N/A Status: Enrolling by invitation
Date: 2024-08-20