| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| 5g |
|
||
| 10g |
|
||
| 25g |
|
||
| Other Sizes |
| 靶点 |
Angiotensin-converting enzyme (ACE)
|
|---|---|
| 体外研究 (In Vitro) |
将血管紧张素 I (ATI) 转变为血管紧张素 II (ATII) 的酶称为血管紧张素转换酶 (ACE),赖诺普利二水合物是该酶的强效竞争性抑制剂。肾素-血管紧张素-醛固酮系统 (RAAS) 成分 ATII 控制血压。赖诺普利用于治疗高血压、症状性充血性心力衰竭、延长某些患者心肌梗死后的生存期,以及阻止患有糖尿病、微量白蛋白尿或明显肾病的高血压患者肾病的进展[1][2]。
|
| 体内研究 (In Vivo) |
与未经治疗的自发性高血压大鼠 (SHR) 大鼠 (+27%) 相比,赖诺普利治疗的 SHR 大鼠的总胆固醇水平显着升高,但与赖诺普利治疗的 Wistar京都大鼠 (WKY) 大鼠相比没有显着升高。赖诺普利是一种长效血管紧张素转换酶抑制剂,可阻断肾素-血管紧张素系统 (RAS) 并降低大鼠的全身血压。赖诺普利降低大鼠治疗组(百草枯 + 赖诺普利)和治疗组(赖诺普利 + 百草枯)肺组织中的羟脯氨酸水平并抑制胶原蛋白的积累。赖诺普利可维持大鼠的超滤量 (UF)、葡萄糖重吸收 (D 1 /D 0 葡萄糖) 和腹膜厚度。 Lisinopril(0.2 mg/kg,每天两次,持续 10 天)可保护豚鼠心脏细胞膜的完整性并减轻自由基诱导的氧化应激。
|
| 酶活实验 |
酶活性测定[1]
s-ACE活性测定采用2-呋喃丙烯酰- 1 -苯丙酰酰甘氨酸(FAPGG)作为底物,如前所述。简单地说,用50 mM HEPES, 0.3 M NaCl和10 μM ZnCl2在pH 7.5下缓冲蛋白质。将770微升缓冲液与200 μL 0.5 mM FAPGG混合。将30 μL含1-3 μg蛋白质的酶溶液加入恒温25℃的培养皿中,开始反应。在334 nm处监测吸光度下降2分钟,并测定初始速度。一个单位的活性被定义为产生1.0的−ΔA 334/min的s-ACE的量。纯化后的s-ACE活性为35 ~ 37 U/mg。在恒定抑制剂浓度和不同底物浓度下进行活性测定,以确定抑制常数。 等温滴定量热法[1] ITC实验采用MCS微热量计进行。 参考池充满水,仪器使用标准电脉冲进行校准。用循环水浴来稳定温度。这仪器被允许在一夜之间达到平衡。所有溶液在使用前在真空下搅拌彻底脱气。每隔6 min, 10 μl等量滴入s-ACE溶液。注射注射器,在其上安装一个桨,搅拌溶液在300 rpm,确保立即混合。用于滴定的蛋白浓度为3.5 ~ 16.4 μM,抑制剂浓度为0.6 ~ 3.0 mM (l-Asp-l-Phe)和0.2 ~ 0.5 mM (lisinopril/赖诺普利、卡托普利和依那普利)。本研究的所有实验均在NaCl浓度为300 mM和至少两种不同缓冲液中进行。将s-ACE抑制剂等量注射到仅含缓冲液的细胞中进行稀释实验。对热图的峰进行积分,结合反应产生的热量计算为反应热与相应稀释热之差。 |
| 细胞实验 |
最近的研究表明,二肽基肽酶4 (DPP4)抑制剂增加了大疱性类天疱疮(BP)的发展风险,这是最常见的自身免疫性水泡皮肤病;然而,相关机制尚不清楚,到目前为止,还没有确定药物性BP的治疗靶点。因此,我们使用临床数据挖掘来确定可以抑制DPP4抑制剂相关BP的候选药物,并通过人类外周血单个核细胞(hPBMCs)实验研究了潜在的分子机制。对美国食品和药物管理局不良事件报告系统和IBM®MarketScan®研究数据库的搜索表明,DPP4抑制剂增加了BP的风险,同时使用血管紧张素转换酶抑制剂赖诺普利可显著降低接受DPP4抑制剂的患者的BP发生率。此外,hPBMCs体外实验表明,DPP4抑制剂上调了单核/巨噬细胞中与BP病理生理有关的MMP9和ACE2的mRNA表达。此外,赖诺普利和Mas受体(MasR)抑制剂抑制DPP4抑制剂诱导的MMP9上调。这些发现表明肾素-血管紧张素系统的调节,特别是血管紧张素1-7/MasR轴,是DPP4抑制剂相关BP的治疗靶点。[3]
|
| 动物实验 |
In this study, 2 groups from each of the 3 rat strains had their hearts irradiated (8 Gy X 5 fractions). One irradiated group was treated with the ACE-inhibitor lisinopril, and a separate group in each strain served as nonirradiated controls. Radiation reduced cardiac end diastolic volume by 9-11% and increased thickness of the interventricular septum (11-16%) and left ventricular posterior wall (14-15%) in all 3 strains (5-10 rats/group) after 120 days. Lisinopril mitigated the increase in posterior wall thickness. Mitochondrial function was measured by the Seahorse Cell Mitochondrial Stress test in peripheral blood mononuclear cells (PBMC) at 90 days. Radiation did not alter mitochondrial respiration in PBMC from BN or SSBN6. However, maximal mitochondrial respiration and spare capacity were reduced by radiation in PBMC from SS rats (p=0.016 and 0.002 respectively, 9-10 rats/group) and this effect was mitigated by lisinopril (p=0.04 and 0.023 respectively, 9-10 rats/group). Taken together, these results indicate injury to the heart by radiation in all 3 strains of rats, although the SS rats had greater susceptibility for mitochondrial dysfunction. Lisinopril mitigated injury independent of genetic background.[4]
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Lisinopril is 6-60% orally bioavailable with an average of 25% bioavailability. Lisinopril reaches a Cmax of 58ng/mL with a Tmax of 6-8h. Lisinopril's absorption is not affected by food. Lisinopril is entirely eliminated exclusively in the urine. The apparent volume of distribution of lisinopril is 124L. A 30kg child has a typical clearance of 10L/h, which increases with renal function. The mean renal clearance of lisinopril in healthy adult males is 121mL/min. Steady state is attained after two daily doses (every 24 hours) in healthy volunteers. The drug is not metabolized but is eliminated via the kidneys. In dogs, lisinopril's bioavilability ranges from 24-50% with peak levels occurring approximately 4 hours after dosing. Lisinopril is distributed poorly into the CNS. It is unknown if it is distributed into maternal milk, but it does cross the placenta. Following oral administration of Prinivil, peak serum concentrations of lisinopril occur within about 7 hours, although there was a trend to a small delay in time taken to reach peak serum concentrations in acute myocardial infarction patients. Declining serum concentrations exhibit a prolonged terminal phase which does not contribute to drug accumulation. This terminal phase probably represents saturable binding to ACE and is not proportional to dose. Lisinopril does not appear to be bound to other serum proteins. Lisinopril does not undergo metabolism and is excreted unchanged entirely in the urine. Based on urinary recovery, the mean extent of absorption of lisinopril is approximately 25 percent, with large inter-subject variability (6-60 percent) at all doses tested (5-80 mg). Lisinopril absorption is not influenced by the presence of food in the gastrointestinal tract. The absolute bioavailability of lisinopril is reduced to about 16 percent in patients with stable NYHA Class II-IV congestive heart failure, and the volume of distribution appears to be slightly smaller than that in normal subjects. The oral bioavailability of lisinopril in patients with acute myocardial infarction is similar to that in healthy volunteers. For more Absorption, Distribution and Excretion (Complete) data for LISINOPRIL (9 total), please visit the HSDB record page. Metabolism / Metabolites Lisinopril is not metabolized and is excreted as the unchanged drug. Lisinopril does not undergo metabolism and is excreted unchanged entirely in the urine. Biological Half-Life Lisinopril has an effective half life of accumulation of 12.6h and a terminal half life of 46.7h. The plasma half-life controlling accumulation during chronic administration is 12-13 hr and the absorbed drug is eliminated via glomerular filtration. The accumulation half-life averages 12.6 hours despite a terminal serum half-life of approximately 40 hours /in healthy volunteers/. Upon multiple dosing, lisinopril exhibits an effective half-life of 12 hours. |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION AND USE: Lisinopril is angiotensin-converting enzyme (ACE) inhibitor, antihypertensive and cardiotonic agent. HUMAN EXPOSURE AND TOXICITY: The most likely manifestation of overdosage would be hypotension, for which the usual treatment would be intravenous infusion of normal saline solution. Marked hypotension may occur in patients with congestive heart failure-potential for myocardial infarction or stroke in those with acute myocardial infarction or ischemic cardiovascular or cerebrovascular disease. Rare ACE inhibitor-associated clinical syndrome manifested initially by cholestatic jaundice or hepatitis; may progress to fulminant hepatic necrosis and is potentially fatal. Patients receiving an ACE inhibitor, including lisinopril, who develop jaundice or marked elevations in hepatic enzymes should discontinue the drug and receive appropriate monitoring. Hyperkalemia can develop, especially in those with renal impairment or diabetes mellitus and those receiving drugs that can increase serum potassium concentration. Sensitivity reactions, including anaphylactoid reactions and angioedema (including laryngeal edema, tongue edema), are potentially fatal. Use of drugs that act on the renin-angiotensin system during the second and third trimesters of pregnancy reduces fetal renal function and increases fetal and neonatal morbidity and death. ANIMAL STUDIES: There was no evidence of a tumorigenic effect when lisinopril was administered for 105 weeks to male and female rats at doses up to 90 mg per kg per day or for 92 weeks to male and female mice at doses up to 135 mg per kg per day. Lisinopril treatment in male rats resulted in a marked decrease in sperm density, sperm motility and zona pellucida penetration. Acrosome reaction by spermatozoa obtained from drug-treated animals was significantly lower when compared with spermatozoa from normal animals. The developmental toxicity of lisinopri have been tested in mice and rabbits. In mice, the incidence of resorptions increased at all dosage levels. No treatment-related adverse effects were found on litter sizes of live fetuses and mean fetal weights. In rabbits, mean fetal weights were normal, although ossification was retarded at all dosage levels. External, skeletal and visceral examinations did not reveal any teratogenic potential at any dosage level in mice and rabbits. Lisinopril was not mutagenic in the Ames microbial mutagen test with or without metabolic activation. It was also negative in a forward mutation assay using Chinese hamster lung cells. Lisinopril did not produce single strand DNA breaks in an in vitro alkaline elution rat hepatocyte assay. In addition, lisinopril did not produce increases in chromosomal aberrations in an in vitro test in Chinese hamster ovary cells or in an in vivo study in mouse bone marrow. There were 1781 dogs exposed to lisinopril and 156 that became symptomatic. The most common clinical signs included: lethargy (24%), tachycardia (18%), vomiting (14%) and hypotension (13%). Of the 98 cats, 7 were symptomatic with 29% hypertensive 29% tachycardic and 29% vomiting. Interactions Potential pharmacologic interaction (additive hyperkalemic effect). Includes potassium-sparing diuretics, potassium supplements, and other drugs that can increase serum potassium. The manufacturer states that lisinopril should be used cautiously (with frequent monitoring of serum potassium), if at all, with potassium supplements or salt substitutes containing potassium. Potential pharmacologic interaction (increased hypoglycemic effect), especially during initial weeks of combined treatment /of lisinopril and antidiabetic agents/ and in patients with renal impairment. Potential pharmacologic interaction (additive hyperkalemic effect). Includes potassium-sparing diuretics, potassium supplements, and other drugs that can increase serum potassium. The manufacturer states that lisinopril should be used cautiously (with frequent monitoring of serum potassium), if at all, with potassium supplements or salt substitutes containing potassium. Potential pharmacologic interaction (decreased antihypertensive effect) when lisinopril is used concomitantly concurrently with nonsteroidal anti-inflammatory agents (NSAIAs). Potential pharmacologic interaction (decreased renal function) when lisinopril is used concomitantly with NSAIAs in patients with impaired renal function. For more Interactions (Complete) data for LISINOPRIL (14 total), please visit the HSDB record page. |
| 参考文献 |
|
| 其他信息 |
Therapeutic Uses
Angiotensin-Converting Enzyme Inhibitors; Antihypertensive Agents; Cardiotonic Agents Prinivil is indicated for the treatment of hypertension in adult patients and pediatric patients 6 years of age and older to lower blood pressure. Lowering blood pressure lowers the risk of fatal and non-fatal cardiovascular events, primarily strokes and myocardial infarctions. ... Prinivil may be administered alone or with other antihypertensive agents /Included in US product labeling/ Prinivil is indicated to reduce signs and symptoms of heart failure in patients who are not responding adequately to diuretics and digitalis /Included in US product labeling/ Prinivil is indicated for the reduction of mortality in treatment of hemodynamically stable patients within 24 hours of acute myocardial infarction. Patients should receive, as appropriate, the standard recommended treatments such as thrombolytics, aspirin and beta-blockers. /Included in US product label/ For more Therapeutic Uses (Complete) data for LISINOPRIL (6 total), please visit the HSDB record page. Drug Warnings /BOXED WARNING/ WARNING: FETAL TOXICITY When pregnancy is detected, discontinue Prinivil as soon as possible. Drugs that act directly on the renin-angiotensin system can cause injury and death to the developing fetus. Milk of lactating rats contains radioactivity following administration of (14)C lisinopril. It is not known whether this drug is secreted in human milk. Because many drugs are secreted in human milk, and because of the potential for serious adverse reactions in nursing infants from ACE inhibitors, discontinue nursing or discontinue Prinivil. Antihypertensive effects and safety of Prinivil have been established in pediatric patients aged 6 to 16 years. No relevant differences between the adverse reaction profile for pediatric patients and adult patients were identified. Safety and effectiveness of Prinivil have not been established in pediatric patients under the age of 6 or in pediatric patients with glomerular filtration rate <30 mL/min/1.73 sq m. Adverse effects reported in greater than 1% of patients receiving lisinopril for the management of heart failure and more frequently than with placebo include dizziness, hypotension, headache, diarrhea, chest pain, nausea, abdominal pain, rash, and upper respiratory tract infection. For more Drug Warnings (Complete) data for LISINOPRIL (23 total), please visit the HSDB record page. Pharmacodynamics Lisinopril is an angiotensin converting enzyme inhibitor used to treat hypertension, heart failure, and myocardial infarction. Lisinopril is not a prodrug, and functions by inhibition of angiotensin converting enzyme as well as the renin angiotensin aldosterone system. It has a wide therapeutic index and a long duration of action as patients are generally given 10-80mg daily. |
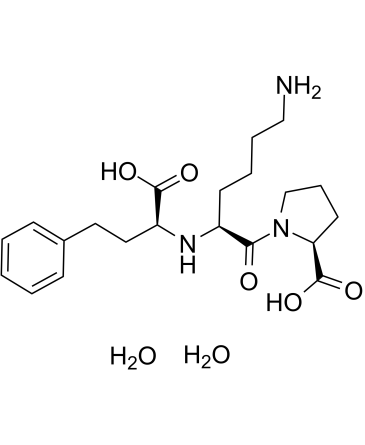
| 分子式 |
C21H35N3O7
|
|---|---|
| 分子量 |
441.52
|
| 精确质量 |
441.247
|
| 元素分析 |
C, 57.13; H, 7.99; N, 9.52; O, 25.37
|
| CAS号 |
83915-83-7
|
| 相关CAS号 |
Lisinopril;76547-98-3;Lisinopril-d5;1356905-39-9
|
| PubChem CID |
5362119
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.251 g/cm3
|
| 沸点 |
666.4ºC at 760 mmHg
|
| 熔点 |
160ºC (Decomposes)
|
| 闪点 |
356.9ºC
|
| 折射率 |
-45 ° (C=1, 0.25mol/L Zinc Acetate Buffer)
|
| LogP |
2.264
|
| tPSA |
132.96
|
| 氢键供体(HBD)数目 |
4
|
| 氢键受体(HBA)数目 |
7
|
| 可旋转键数目(RBC) |
12
|
| 重原子数目 |
29
|
| 分子复杂度/Complexity |
550
|
| 定义原子立体中心数目 |
3
|
| SMILES |
C1C[C@H](N(C1)C(=O)[C@H](CCCCN)N[C@@H](CCC2=CC=CC=C2)C(=O)O)C(=O)O
|
| InChi Key |
CZRQXSDBMCMPNJ-ZUIPZQNBSA-N
|
| InChi Code |
InChI=1S/C21H31N3O5.2H2O/c22-13-5-4-9-16(19(25)24-14-6-10-18(24)21(28)29)23-17(20(26)27)12-11-15-7-2-1-3-8-15/h1-3,7-8,16-18,23H,4-6,9-14,22H2,(H,26,27)(H,28,29)2*1H2/t16-,17-,18-/m0../s1
|
| 化学名 |
((S)-1-carboxy-3-phenylpropyl)-L-lysyl-L-proline dihydrate
|
| 别名 |
MK-521; MK521; MK 521; Lisinopril dihydrate; Lisinopril dihydrate; 83915-83-7; Renacor; Lisinopril (dihydrate); MK-521; CHEBI:6503; E7199S1YWR; Prinivil; Qbrelis; Ranolip; Renacor
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
H2O : ~33.33 mg/mL (~75.49 mM)
DMSO : ~1 mg/mL (~2.26 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 50 mg/mL (113.25 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶。
请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.2649 mL | 11.3245 mL | 22.6490 mL | |
| 5 mM | 0.4530 mL | 2.2649 mL | 4.5298 mL | |
| 10 mM | 0.2265 mL | 1.1325 mL | 2.2649 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。