| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| Other Sizes |
|
| 靶点 |
HDAC5 ( IC50 = 4.22 nM ); HDAC4 ( IC50 = 11.9 nM ); HDAC6 ( IC50 = 55.7 nM ); HDAC1 ( IC50 = 320 nM ); HDAC11 ( IC50 = 852 nM ); HDAC2 ( IC50 = 881 nM ); HDAC8 ( IC50 = 1278 nM )
|
|---|---|
| 体外研究 (In Vitro) |
体外活性:LMK-235 在对顺铂具有不同敏感性的人类癌细胞系中引起 HDAC 抑制,IC50 <1 μM。在乳腺癌细胞系MDA-MB-231、舌癌细胞系Cal27和食道细胞系Kyse510细胞系中,LMK-235表现出高细胞毒性,并显着增强顺铂的细胞毒性。此外,LMK-235 还表现出针对多个疟疾寄生虫生命周期阶段的纳摩尔活性。激酶测定:根据公司的标准操作程序,通过基于荧光的测定来进行化合物对七种人类 HDAC 亚型(1、2、4 C2A、5 C2A、6、8 和 11)的体外抑制活性。 IC50 值是使用 10 个不同浓度从 10 μM 开始进行 3 倍连续稀释来确定的。 TSA 和伏立诺他用作参考化合物。细胞测定:通过改进的MTT测定评估测试物质作用下的细胞存活率。该测定基于活细胞将黄色 MTT 代谢为紫色甲臜的能力,可通过分光光度法检测。简而言之,A2780、Cal27、Kyse510 和 MDA-MB-231 细胞系以 5000、7000、8000 和 10000 个细胞/孔的密度接种在 96 孔板中。 24小时后,将细胞暴露于浓度增加的测试化合物中。 72 小时后结束孵育,通过添加 MTT 溶液(5 mg/mL,磷酸盐缓冲盐水)测定细胞存活率。将甲臜沉淀溶解在 DMSO 中。在 FLUOstar 酶标仪中测量 544 和 690 nm 处的吸光度。
|
| 体内研究 (In Vivo) |
在小鼠体内研究中,在增殖测定和集落形成测定中证明了 LMK235 和阿糖胞苷的协同抑制作用。这些发现表明体内RNAi筛选治疗效果是可行的。 HDAC4可能是增强抗白血病药物疗效的重要靶点。
|
| 酶活实验 |
根据该公司的标准操作程序,使用基于荧光的测定评估化合物对七种人类 HDAC 亚型(1、2、4 C2A、5 C2A、6、8 和 11)的体外抑制活性。使用三倍连续稀释和十种不同浓度(从 10 μM 开始)计算 IC50 值。伏立诺他和 TSA 是参考化合物。
全细胞HDAC抑制试验[1] 细胞HDAC测定是基于Ciossek等人和Bonfils等人发表的一项测定,并进行了轻微修改。 简单地说,将人癌细胞系Cal27sens/Cal27 CisR、Kyse510sens/Kyse510 CisR、A2780/A2780 CisR和MDA-MB231sens/CisR以1.5 × 104个/孔的密度接种于96孔组织培养板中,培养液总量为90 μL。24 h后,细胞孵养18 h,增加测试化合物浓度,如Compound 19i (LMK235)。加入10 μL 3 mM的Boc-Lys(ε-Ac)-AMC,反应至终浓度为0.3 mM,细胞与Boc-Lys(ε-Ac)-AMC在细胞培养条件下孵育3 h。孵育后,加入100 μL/孔停液(25 mM Tris-HCl (pH 8)、137 mM NaCl、2.7 mM KCl、1 mM MgCl2、1% NP40、2.0 mg/mL胰蛋白酶、10 μM vorinostat),在细胞培养条件下孵育3 h。在NOVOstar微孔板读取仪上测量激发波长为320 nm、发射波长为520 nm的荧光强度。 HDAC IC50分析[1] 化合物19e、19h和化合物19i (LMK235)对7种人类HDAC亚型(1,2,4 C2A, 5 C2A, 6, 8和11)的体外抑制活性根据公司的标准操作程序进行荧光检测。采用10种不同浓度测定IC50值,从10 μM开始进行3倍连续稀释。以TSA和伏立诺他为对照化合物。 |
| 细胞实验 |
改进的MTT测定用于确定在测试物质作用下细胞的存活率。该测定是基于活细胞将黄色 3-(4,5-二甲基噻唑-2-基)-2,5-二苯基溴化四唑 (MTT) 转化为紫色甲臜(可通过分光光度法测量)的能力来实现的。简而言之,96 孔板中每孔接种 5000、7000、8000 和 10,000 个细胞的 A2780、Cal27、Kyse510 和 MDA-MB-231 细胞系。 24 小时后,细胞暴露于更高浓度的测试化合物。 72 小时孵育期后,添加 MTT 溶液(磷酸盐缓冲盐水中 5 mg/mL)用于评估细胞存活率。在 DMSO 中,甲臜沉淀溶解。 FLUOstar 酶标仪在 544 和 690 nm 处测量吸光度[1]。
海马原代神经元与治疗[3] 从P1 Cdkl5 -/Y (n = 4)和Cdkl5 +/Y雄性(n = 4)小鼠中制备海马神经元。简单地说,在解剖显微镜下从小鼠大脑中解剖海马,在37°C下用胰蛋白酶处理15分钟,在室温下用DNase处理2分钟,然后用火抛光玻璃移液器机械研磨以获得单细胞悬液。将大约1.2 × 105个细胞在6孔板上涂有聚l -赖氨酸的盖片上,并在添加B27和谷氨酰胺的Neurobasal培养基中培养。细胞在体外37°C保存于5% co2腐殖化培养箱中,并于镀后第10天固定用于免疫染色或western blot分析(DIV10)。Compound 19i (LMK235)从DIV2开始隔天给药。 |
| 动物实验 |
C57BL/6-BALB/c mice
20 mg/kg i.p. Colony and treatments[3] Mice for experiments were produced by crossing Cdkl5 +/− females with Cdkl5 -/Y males and Cdkl5 +/− females with Cdkl5 +/Y males; animals were genotyped by PCR of genomic DNA as previously described and littermate controls were used for all experiments. The day of birth was designated postnatal day (P) zero and animals that were 24 h of age were considered as 1-day-old animals (P1). After weaning, mice were housed 3 to 5 per cage on a 12-h light/dark cycle in a temperature-and humidity-controlled environment with food and water provided ad libitum.[3] Starting from postnatal day 40 (P40), Cdkl5 +/Y and Cdkl5 -/Y male mice were treated with vehicle (PBS) or Compound 19i (LMK235) (N-((6 (hydroxyamino)-6-oxohexyl)oxy)-3,5-dimethylbenzamide) 5 mg/kg or 20 mg/kg administered i.p. daily for 8 or 16 days. Animals were sacrificed on P48 or P56. |
| 参考文献 |
|
| 其他信息 |
The synthesis and biological evaluation of new potent hydroxamate-based HDAC inhibitors with a novel alkoxyamide connecting unit linker region are described. Biological evaluation includes MTT and cellular HDAC assays on sensitive and chemoresistant cancer cell lines as well as HDAC profiling of selected compounds. Compound 19i (LMK235) (N-((6-(hydroxyamino)-6-oxohexyl)oxy)-3,5-dimethylbenzamide) showed similar effects compared to vorinostat on inhibition of cellular HDACs in a pan-HDAC assay but enhanced cytotoxic effects against the human cancer cell lines A2780, Cal27, Kyse510, and MDA-MB231. Subsequent HDAC profiling yielded a novel HDAC isoform selectivity profile of 19i in comparison to vorinostat or trichostatin A (TSA). 19i shows nanomolar inhibition of HDAC4 and HDAC5, whereas vorinostat and TSA inhibit HDAC4 and HDAC5 in the higher micromolar range.[1]
Purpose: Histone deacetylase 5 (HDAC5) is an important protein in neural and cardiac diseases and a potential drug target. However, little is known regarding the specific role of HDAC5 in breast cancer (BC). We aimed to evaluate HDAC5 expression in human breast tumors and to determine the effects of the inhibition of HDAC5 expression in BC cells. Experimental design: HDAC5 expression was evaluated in BC patients and was correlated with clinical features and with patient prognosis. Functional experiments were performed using shRNA and the selective HDAC inhibitor LMK-235 for HDAC5 knockdown and inhibition in BC cells. The synergistic effects of LMK-235 with the proteasome inhibitor bortezomib were also examined. Results: HDAC5 was extensively expressed in human BC tissues, and high HDAC5 expression was associated with an inferior prognosis. Knockdown of HDAC5 inhibited cell proliferation, migration, invasion, and enhanced apoptosis. The HDAC5 inhibitor LMK-235 inhibited cell growth and induced apoptosis, while the inclusion of bortezomib synergistically enhanced the efficacy of LMK-235. Conclusions: Our findings indicate that HDAC5 is a promising prognostic marker and drug target for BC and that the combination of LMK-235 and bortezomib presents a novel therapeutic strategy for BC.[2] Cyclin-dependent kinase-like 5 (CDKL5) is a Ser/Thr protein kinase predominantly expressed in the brain. Mutations of the CDKL5 gene lead to CDKL5 disorder, a neurodevelopmental pathology that shares several features with Rett Syndrome and is characterized by severe intellectual disability. The phosphorylation targets of CDKL5 are largely unknown, which hampers the discovery of therapeutic strategies for improving the neurological phenotype due to CDKL5 mutations. Here, we show that the histone deacetylase 4 (HDAC4) is a direct phosphorylation target of CDKL5 and that CDKL5-dependent phosphorylation promotes HDAC4 cytoplasmic retention. Nuclear HDAC4 binds to chromatin as well as to MEF2A transcription factor, leading to histone deacetylation and altered neuronal gene expression. By using a Cdkl5 knockout (Cdkl5 -/Y) mouse model, we found that hypophosphorylated HDAC4 translocates to the nucleus of neural precursor cells, thereby reducing histone 3 acetylation. This effect was reverted by re-expression of CDKL5 or by inhibition of HDAC4 activity through the HDAC4 inhibitor LMK235. In Cdkl5 -/Y mice treated with LMK235, defective survival and maturation of neuronal precursor cells and hippocampus-dependent memory were fully normalized. These results demonstrate a critical role of HDAC4 in the neurodevelopmental alterations due to CDKL5 mutations and suggest the possibility of HDAC4-targeted pharmacological interventions.[3] |
| 分子式 |
C15H22N2O4
|
|
|---|---|---|
| 分子量 |
294.35
|
|
| 精确质量 |
294.157
|
|
| 元素分析 |
C, 61.21; H, 7.53; N, 9.52; O, 21.74
|
|
| CAS号 |
1418033-25-6
|
|
| 相关CAS号 |
|
|
| PubChem CID |
71520717
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 密度 |
1.2±0.1 g/cm3
|
|
| 折射率 |
1.538
|
|
| LogP |
2.05
|
|
| tPSA |
94.64
|
|
| 氢键供体(HBD)数目 |
3
|
|
| 氢键受体(HBA)数目 |
4
|
|
| 可旋转键数目(RBC) |
8
|
|
| 重原子数目 |
21
|
|
| 分子复杂度/Complexity |
326
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
O(C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C(N([H])O[H])=O)N([H])C(C1C([H])=C(C([H])([H])[H])C([H])=C(C([H])([H])[H])C=1[H])=O
|
|
| InChi Key |
VRYZCEONIWEUAV-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C15H22N2O4/c1-11-8-12(2)10-13(9-11)15(19)17-21-7-5-3-4-6-14(18)16-20/h8-10,20H,3-7H2,1-2H3,(H,16,18)(H,17,19)
|
|
| 化学名 |
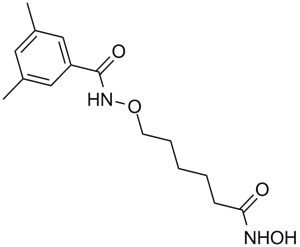
N-[6-(hydroxyamino)-6-oxohexoxy]-3,5-dimethylbenzamide
|
|
| 别名 |
LMK 235; LMK235; N-((6-(hydroxyamino)-6-oxohexyl)oxy)-3,5-dimethylbenzamide; LMK235; N-[6-(hydroxyamino)-6-oxohexoxy]-3,5-dimethylbenzamide; CHEMBL2312168; 6-{[(3,5-dimethylphenyl)formamido]oxy}-N-hydroxyhexanamide; LMK-235
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (7.07 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (7.07 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (7.07 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 5%DMSO+ 40%PEG300+ 5%Tween 80+ 50%ddH2O: 3.0mg/ml (10.19mM) 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.3973 mL | 16.9866 mL | 33.9732 mL | |
| 5 mM | 0.6795 mL | 3.3973 mL | 6.7946 mL | |
| 10 mM | 0.3397 mL | 1.6987 mL | 3.3973 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。

Hyperacetylation of P. falciparum histones. (Compound 1a is LMK-235)Eur J Med Chem.2014 Jul 23;82:204-13. |
|---|
LMK-235 synergizes with bortezomib in BC cells.Oncotarget.2016 Jun 21;7(25):37966-37978. |
LMK-235 inhibits BC cell proliferation and induces apoptosis.Oncotarget.2016 Jun 21;7(25):37966-37978. |