| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| Other Sizes |
|

| 靶点 |
H1 receptor; B(0)AT2 ( IC50 = 4 μM )
|
||
|---|---|---|---|
| 体外研究 (In Vitro) |
体外活性:氯雷他定被确定为 B(0)AT2 的选择性抑制剂,IC50 为 4 μM,同时对 SLC6 家族的其他几个成员活性较低或无活性。在人 Fc epsilon RI+ 细胞中,在 Der p 1 抗原或抗 Fc epsilon RI 攻击之前预孵育时,氯雷他定浓度依赖性地抑制组胺和 LTC4 的释放。 Loratadine (0.1 mM) 还可抑制 (10-40%) 组胺、LTC4 和 PGD2 从抗 Fc epsilon RI 激活的纯化 HLMC (16-68%) 中释放。氯雷他定对用抗 Fc epsilon RI 进行免疫攻击的纯化 HSMC (24-72%) 中的组胺、类胰蛋白酶、LTC4 和 PGD2 释放产生浓度依赖性抑制 (10-40%)。 Loratadine 显着抑制组胺诱导的 IL-6 和 IL-8 分泌,并在人脐静脉内皮细胞 (HUVEC) 中具有更强大的活性代谢物功效。氯雷他定以浓度、电压、时间和使用依赖性方式阻断hKv1.5通道,但仅在浓度远高于用hKv1.5通道编码基因转染的Ltk细胞中的治疗血浆水平时进行。 Loratadine 可抑制鼻病毒诱导的原代支气管或转化 (A549) 呼吸道上皮细胞中 ICAM-1 的上调。氯雷他定还以剂量依赖性方式抑制鼻病毒感染引起的 ICAM-1 mRNA 诱导,并且完全抑制鼻病毒诱导的 ICAM-1 启动子激活。细胞测定:氯雷他定 (SCH-29851) 是一种选择性反向外周组胺 H1 受体激动剂,IC50 > 32 μM。氯雷他定是一种非镇静性抗组胺药,可抑制组胺诱导的内皮细胞分泌 IL-6 和 IL-8 的活性。
|
||
| 动物实验 |
|
||
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Loratadine is rapidly absorbed and achieves peak plasma concentration in 1-2 hours, while it's main metabolite achieves peak plasma concentration in 3-4 hours. In the rapid dissolve formulation, the pharmacokinetic parameters of loratadine are as follows: Cmax = 2.56 ng/ml, Tmax = 1.14 hrs, AUC = 6.14 ng x hr/ml. In the rapid dissolve formulation, the pharmacokinetic parameters of descarboethoxyloratadine are as follows: Cmax = 3.72 ng/ml, Tmax = 1.97 hr, AUC = 49.1 ng x hr/ml. In the conventional formulation, the pharmacokinetic parameters of loratadine are as follows: Cmax = 2.11 ng/ml, Tmax = 1.00 hr, AUC = 4.64 ng x hr/ml In the conventional formulation, the pharmacokinetic parameters of descarboethoxyloratadine are as follows: Cmax = 3.66 ng/ml, Tmax = 1.97 hr, AUC = 48.4 ng x hr/ml Over a 10 day period, 40% of loratadine is excreted in the urine, and 42% is eliminated in the faeces. The volume of distribution of loratadine is 120 L/Kg. The clearance of loratadine after single oral doses of 20 mg and 40 mg are 12 L/h/kg and 9 L/h/kg respectively. P-glycoprotein is involved in the clearance of many 2nd generation antihistamines, including loratadine, from the central nervous system. 1st generation antihistamines are not cleared by P-glycoprotein, which may help explain why they have a different central nervous system adverse effect profile compared to their 2nd generation counterparts. It appears that an antihistamine with higher affinity for p-glycoprotein will have a lower incidence of CNS adverse effects. H1 antagonists are eliminated more rapidly by children than by adults and more slowly in those with severe liver disease. /H1 Receptor Antagonists/ The H1 antagonists are well absorbed from the gastrointestinal tract. Following oral administration, peak plasma concentrations are achieved in 2 to 3 hours ... . /H1 Receptor Antagonists/ Approximately 80% of the total dose administered can be found equally distributed between urine and feces in the form of metabolic products after 10 days. Whole body autoradiographic studies in rats and monkeys, radiolabeled tissue distribution studies in mice and rats, and in vivo radioligand studies in mice have shown that neither loratadine nor its metabolites readily cross the blood-brain barrier. Radioligand binding studies with guinea pig pulmonary and brain H1-receptors indicate that there was preferential binding to peripheral versus central nervous system H1-receptors. Unlike other currently available antihistamines, second generation antihistamines such as ... loratadine appear to distribute poorly or not appreciably into the CNS at usual dosages. Metabolism / Metabolites Loratadine undergoes extensive first pass metabolism in the liver and is primarily metabolized by CYP3A4, CYP2D6, CYP1A1 and CYP2C19. Less involved CYP enzymes include CYP1A2, CYP2B6, CYP2C8, CYP2C9 and CYP3A5. CYP3A4 and CYP2D6 are mainly responsible for metabolizing loratadine to descarboethoxyloratadine. This primary metabolite is 4 times more pharmacologically active than loratadine. In addition, a study demonstrates that descarboethoxyloratadine is first glucuronidated by UGT2B10, then hydroxylated by CYP2C8 to form 3-hydroxydesloratadine. Further glucuronidation of 3-hydroxydesloratadine facilitates excretion. The second generation H1 antagonists astemizole, loratadine,and terfenadine are rapidly absorbed from the gastrointestinal tract and metabolized in the liver to active metabolites by the hepatic microsomal p450 system. Pharmacokinetic studies following single and multiple oral doses of loratadine in 115 volunteers showed that loratadine is rapidly absorbed and extensively metabolized to an active metabolite (descarboethoxyloratadine). In vitro studies with human liver microsomes indicate that loratadine is metabolized to descarboethoxyloratadine predominately by p450 CYP3A4 and, to a lesser extent, by p450 CYP2D6. H1 receptor antagonists are among the many drugs that induce hepatic microsomal enzymes, and they may facilitate their own metabolism. /H1 Receptor Antagonists/ The non-sedating anti-histamine, loratadine ... was admin orally in the diet to mature male rats at dosages of 4, 10 and 25 mg/kg/day for 2 wk. The effects of these treatments on liver microsomal cytochrome P450 were evaluated by immunochemical and biochemical techniques, and were compared with the effects of treating rats with three different inducers of cytochrome P450, namely phenobarbital, 3-methylcholanthrene and dexamethasone. Treatment of rats with loratadine caused a dose dependent incr in the levels of P450 2Bl and 2B2, the major phenobarbital inducible P450 enzymes, as determined by Western immunoblotting. At the highest dosage tested, loratadine was less effective than phenobarbital as an inducer of 2Bl and 2B2, although the induction of these proteins could be detected immunochemically even at the lowest dosage of loratadine tested. Consistent with these observations, treatment of rats with loratadine caused a dose dependent incr in the rate of two reactions that are catalyzed predominantly by 2Bl/2, namely testosterone 16 beta-hydroxylation and 7-pentoxyresorufin O-dealkylation. At the highest dosage tested, loratadine caused a 7.3- and 8.5-fold incr in the rate of testosterone 16 beta-hydroxylation and 7-pentoxyresorufin O-dealkylation, respectively, compared with a and 45-fold incr caused by phenobarbital treatment. Treatment of rats with loratadine caused a 1.4 to 2.0-fold incr in the 2 beta-, 6 beta- and 15 beta-hydroxylation of testosterone, which was associated with a similar incr in the levels of immunoreactive P450 3Al and/or 3A2. As an inducer of P450 3Al/2, loratadine was slightly less effective than phenobarbital, and was considerably less effective than dexamethasone, which caused a 10- to 33-fold increase in testosterone 2 beta-, 6 beta- and 15 beta-hydroxylase activity. At the dosages tested, loratadine did not increase the levels of P450 lAl, the major 3-methylcholanthrene inducible P450 enzyme, as determined by Western immunoblotting. The rate of 7-ethoxyresorufin O-dealkylation, which is catalyzed predominantly by P450 lAl, incr 1.9-fold after loratidine treatment, but this incr was less than that caused by phenobarbital treatment (2.2-fold), and was considerably less than that caused by 3-methylcholanthrene treatment (33-fold). The effects of treating mature male mice with loratadine on liver microsomal cytochrome P450 resembled the effects observed in rats. These results indicate that loratadine is a phenobarbital type inducer of liver microsomal cytochrome P450 in rats and mice. Loratadine has known human metabolites that include Desloratadine. Hepatic Half Life: 8.4 hours Biological Half-Life The elimination half life is approximately 10 hours for loratadine and 20 hours for descarboethoxyloratadine. The mean elimination half-lives found in studies in normal adult subjects (n= 54) were 8.4 hours (range= 3 to 20 hours) for loratadine and 28 hours (range= 8.8 to 92 hours) for the major active metabolites (descarboethoxyloratadine). |
||
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation Because of its lack of sedation and low milk levels, maternal use of loratadine would not be expected to cause any adverse effects in breastfed infants. Loratadine might have a negative effect on lactation, especially in combination with a sympathomimetic agent such as pseudoephedrine. The British Society for Allergy and Clinical Immunology recommends loratadine at its lowest dose as a preferred choice if an antihistamine is required during breastfeeding. ◉ Effects in Breastfed Infants A survey of 51 mothers who took loratadine during breastfeeding between 1999 and 2001 was conducted by a teratogen information service. Most of the infants were over 2 months old and loratadine was generally taken for one week or less. Two mothers reported minor sedation in their infants, one at 3 days of age and one at 3 months of age. Both mothers were taking a dose of 10 mg daily. Weight gain and psychomotor development were similar to infants in a control group of breastfed infants unexposed to medications. An extension of the study that compared the results of this study (plus one additional patient) to that of a control group of 88 mothers who took a drug known to be safe while breastfeeding. No differences in sedation or any other side effects (p=0.606) in the infant were found between mothers who took loratadine during breastfeeding and those of the control group. ◉ Effects on Lactation and Breastmilk Antihistamines in relatively high doses given by injection can decrease basal serum prolactin in nonlactating women and in early postpartum women. However, suckling-induced prolactin secretion is not affected by antihistamine pretreatment of postpartum mothers. Whether lower oral doses of antihistamines have the same effect on serum prolactin or whether the effects on prolactin have any consequences on breastfeeding success have not been studied. The prolactin level in a mother with established lactation may not affect her ability to breastfeed. One mother out of 51 mothers who took loratadine while nursing reported that she had decreased milk production after taking loratadine 10 mg daily for less than one week at 4 months postpartum. ◈ What is loratadine? Loratadine is an over-the-counter antihistamine. It has been used to treat symptoms of allergic reactions and colds, such as sneezing, runny nose, watery eyes, itchy throat, and hives. Some brand names for loratadine are Claritin® and Alavert®. In the body, loratadine breaks down into another drug called desloratadine. Desloratadine is also sold as a prescription antihistamine under the brand name Clarinex®.Sometimes when people find out they are pregnant, they think about changing how they take their medication, or stopping their medication altogether. However, it is important to talk with your healthcare providers before making any changes to how you take your medication. Your healthcare providers can talk with you about the benefits of treating your condition and the risks of untreated illness during pregnancy. ◈ I take loratadine. Can it make it harder for me to get pregnant? It is not known if loratadine can make it harder to get pregnant. One animal study did not report problems getting pregnant in females exposed to loratadine. ◈ Does taking loratadine increase the chance of miscarriage? Miscarriage is common and can occur in any pregnancy for many different reasons. A study of 161 people taking loratadine during the first trimester of pregnancy did not show an increased chance of miscarriage. ◈ Does taking loratadine increase the chance of birth defects? Every pregnancy starts out with a 3-5% chance of having a birth defect. This is called the background risk. Taking loratadine is not expected to increase the chance of birth defects above the background risk. One early study raised concern about a possible link between loratadine use in pregnancy and hypospadias (a birth defect where the opening of the penis is shifted toward the underside rather than the tip). However, after later studies did not find the same link, the researchers decided that the cases of hypospadias in their original study were most likely due to chance or other factors instead of exposure to loratadine.Other studies of loratadine use during pregnancy have not found an increased chance of any kind of birth defect, including hypospadias. Also, studies have not found that infants with hypospadias were more often exposed to loratadine during pregnancy than infants without hypospadias. ◈ Does taking loratadine in pregnancy increase the chance of other pregnancy-related problems? Loratadine is not expected to increase the chance of pregnancy-related problems, such as preterm delivery (birth before week 37) or low birth weight (weighing less than 5 pounds, 8 ounces [2500 grams] at birth). ◈ Does taking loratadine in pregnancy affect future behavior or learning for the child? Studies have not been done to see if loratadine can cause behavior or learning issues for the child.Breastfeeding while taking loratadine:Loratadine gets into breast milk in small amounts. The amount of loratadine in breastmilk is too low to cause problems for most babies. Loratadine is one of the preferred antihistamines for use during breastfeeding because it is less likely to cause drowsiness (sleepiness) for the person who is breastfeeding or the baby than some other antihistamines. If you suspect the baby has any symptoms (such as being too sleepy), contact the child’s healthcare provider. Be sure to talk to your healthcare provider about all your breastfeeding questions. ◈ If a male takes loratadine, could it affect fertility or increase the chance of birth defects? Studies have not been done to see if loratadine could affect male fertility (ability to get partner pregnant) or increase the chance of birth defects above the background risk. In general, exposures that fathers or sperm donors have are unlikely to increase risks to a pregnancy. For more information, please see the MotherToBaby fact sheet Paternal Exposures at https://mothertobaby.org/fact-sheets/paternal-exposures-pregnancy/. Protein Binding 97 - 99% of the loratadine is bound to plasma proteins. |
||
| 参考文献 | |||
| 其他信息 |
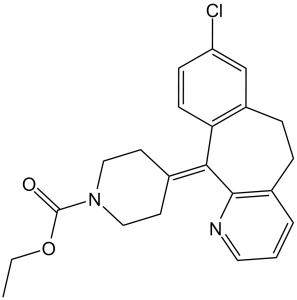
Loratadine is a benzocycloheptapyridine that is 6,11-dihydro-5H-benzo[5,6]cyclohepta[1,2-b]pyridine substituted by a chloro group at position 8 and a 1-(ethoxycarbonyl)piperidin-4-ylidene group at position 11. It is a H1-receptor antagonist commonly employed in the treatment of allergic disorders. It has a role as a geroprotector, a H1-receptor antagonist, an anti-allergic agent and a cholinergic antagonist. It is an ethyl ester, a N-acylpiperidine, a tertiary carboxamide, an organochlorine compound and a benzocycloheptapyridine. It is functionally related to a desloratadine.
Loratadine is a second generation antihistamine used to manage symptoms of allergic rhinitis. A lack of sedative and CNS adverse effects make loratadine, along with other second generation antihistamines, preferable over their 1st generation counterparts in many clinical situations. Loratadine has been reported in Penicillium granulatum with data available. Loratadine is a piperidine histamine H1-receptor antagonist with anti-allergic properties and without sedative effects. Loratadine blocks the H1 histamine receptor and prevents the symptoms that are caused by histamine activity on capillaries, bronchial smooth muscle, and gastrointestinal smooth muscle, including vasodilatation, increased capillary permeability, bronchoconstriction, and spasmodic contraction of gastrointestinal smooth muscle. Loratadine does not cross the blood-brain barrier and does not cause central nervous system effects. Loratadine is a tricyclic antihistamine, which has a selective and peripheral H1-antagonist action. It has a long-lasting effect and does not normally cause drowsiness because it does not readily enter the central nervous system; An antiviral that is used in the prophylactic or symptomatic treatment of influenza A. It is also used as an antiparkinsonian agent, to treat extrapyramidal reactions, and for postherpetic neuralgia. The mechanisms of its effects in movement disorders are not well understood but probably reflect an increase in synthesis and release of dopamine, with perhaps some inhibition of dopamine uptake; Loratadine is a drug used to treat allergies. It is marketed by Schering-Plough under several trade names such as Claritin, Clarityn or Claratyne depending on the market, by Lek as Lomilan and by Wyeth as Alavert. It is also available as a generic; Loratadine is a drug used to treat allergies. It is marketed by Schering-Plough under several trade names such as Claritin, Clarityn or Claratyne depending on the market, by Lek as Lomilan and by Wyeth as Alavert. It is also available as a generic. Its active metabolite, desloratadine, is also on the market, though loratadine itself is the only drug of its class available over the counter (at least in the U.S. as of 2005. Loratadine is available off the shelf in the UK. A second-generation histamine H1 receptor antagonist used in the treatment of allergic rhinitis and urticaria. Unlike most classical antihistamines (HISTAMINE H1 ANTAGONISTS) it lacks central nervous system depressing effects such as drowsiness. See also: Loratadine; pseudoephedrine sulfate (component of); Loratadine hydrochloride (is active moiety of). Drug Indication Loratadine is a 2nd generation antihistamine and is used to manage symptoms of allergic rhinitis, wheal formation, urticaria, and other allergic dermatologic conditions. Mechanism of Action Histamine release is a key mediator in allergic rhinitis and urticaria. As a result, loratadine exerts it's effect by targeting H1 histamine receptors. Loratadine binds to H1 histamine receptors found on the surface of epithelial cells, endothelial cells, eosinophils, neutrophils, airway cells, and vascular smooth muscle cells among others. H1 histamine receptors fall under the wider umbrella of G-protein coupled receptors, and exist in a state of equilibrium between the active and inactive forms. Histamine binding to the H1-receptor facilitates cross linking between transmembrane domains III and V, stabilizing the active form of the receptor. On the other hand, antihistamines bind to a different site on the H1 receptor favouring the inactive form. Hence, loratadine can more accurately be classified as an "inverse agonist" as opposed to a "histamine antagonist", and can prevent or reduce the severity of histamine mediated symptoms. All of the available H1 receptor antagonists are reversible, competitive inhibitors of the interaction of histamine with H1 receptors. /H1 Receptor Antagonists/ H1 antagonists inhibit most responses of smooth muscle to histamine. /H1 Antagonists Receptors/ Within the vascular tree, the H1 antagonists inhibit both the vasoconstrictor effects of histamine and, to a degree, the more rapid vasodilator effects that are mediated by H1 receptors on endothelial cells. /H1 Receptor Antagonists/ H1 antagonists strongly block the action of histamine that results in increased capillary permeability and formation of edema and wheal. /H1 Receptor Antagonists/ For more Mechanism of Action (Complete) data for LORATADINE (6 total), please visit the HSDB record page. |
| 分子式 |
C22H23CLN2O2
|
|
|---|---|---|
| 分子量 |
382.88
|
|
| 精确质量 |
382.144
|
|
| 元素分析 |
C, 69.01; H, 6.05; Cl, 9.26; N, 7.32; O, 8.36
|
|
| CAS号 |
79794-75-5
|
|
| 相关CAS号 |
Loratadine-d4; 381727-27-1; Loratadine-d5; 1398065-63-8
|
|
| PubChem CID |
3957
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 密度 |
1.3±0.1 g/cm3
|
|
| 沸点 |
531.3±50.0 °C at 760 mmHg
|
|
| 熔点 |
134-136°C
|
|
| 闪点 |
275.1±30.1 °C
|
|
| 蒸汽压 |
0.0±1.4 mmHg at 25°C
|
|
| 折射率 |
1.614
|
|
| LogP |
5.94
|
|
| tPSA |
42.43
|
|
| 氢键供体(HBD)数目 |
0
|
|
| 氢键受体(HBA)数目 |
3
|
|
| 可旋转键数目(RBC) |
2
|
|
| 重原子数目 |
27
|
|
| 分子复杂度/Complexity |
569
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
O=C(N1CC/C(=C2/C3C(=CC(=CC=3)Cl)CCC3C/2=NC=CC=3)/CC1)OCC
|
|
| InChi Key |
JCCNYMKQOSZNPW-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C22H23ClN2O2/c1-2-27-22(26)25-12-9-15(10-13-25)20-19-8-7-18(23)14-17(19)6-5-16-4-3-11-24-21(16)20/h3-4,7-8,11,14H,2,5-6,9-10,12-13H2,1H3
|
|
| 化学名 |
ethyl 4-(13-chloro-4-azatricyclo[9.4.0.03,8]pentadeca-1(11),3(8),4,6,12,14-hexaen-2-ylidene)piperidine-1-carboxylate
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (6.53 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (6.53 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (6.53 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 5%DMSO + Corn oil: 4.0mg/ml (10.45mM) 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.6118 mL | 13.0589 mL | 26.1178 mL | |
| 5 mM | 0.5224 mL | 2.6118 mL | 5.2236 mL | |
| 10 mM | 0.2612 mL | 1.3059 mL | 2.6118 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT05421416 | Not yet recruiting | Drug: Loratadine Drug: Placebo |
Stem Cell Transplant Complications |
AHS Cancer Control Alberta | November 1, 2023 | Phase 2 |
| NCT05243706 | Not yet recruiting | Drug: Loratadine Drug: Diosmin/ Hesperidin |
Vinca Alkaloid Adverse Reaction |
Ain Shams University | March 1, 2022 | Phase 3 |
| NCT04211259 | Recruiting | Drug: Loratadine Other: Placebo |
Plasma Cell Myeloma | Rutgers, The State University of New Jersey |
April 18, 2022 | Early Phase 1 |
| NCT06217367 | Recruiting | Drug: 10 mg Loratadine Drug: 5 mg Desloratadine |
Heat Illness Allergic Rhinitis Heat Injury |
Lakehead University | December 5, 2023 | Phase 4 |
| NCT02513290 | Completed | Drug: Bilastine Drug: Loratadine |
Allergic Rhinitis | Universidade do Sul de Santa Catarina |
August 2013 | Phase 4 |
|
|
|