| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| Other Sizes |
|
| 靶点 |
Aldosterone synthase
|
|---|---|
| 体外研究 (In Vitro) |
Lorundrostat选择性抑制人CYP11B2(IC50=9 nM),降低血浆醛固酮和收缩压,可用于肥胖或肾素诱导的高血压研究。
|
| 体内研究 (In Vivo) |
2021年7月至2022年6月,200名参与者被随机分配,最终随访时间为2022年9月。在PRA抑制的参与者接受8周的治疗后,分别使用100 mg、50 mg和12.5 mg的lorundrostat和安慰剂,观察到办公室收缩压的变化为-14.1、-13.2、-6.9和-4.1 mm Hg。在接受每日两次25mg和12.5mg氯霜的个体中,观察到的收缩压降低分别为-10.1和-13.8 mm Hg。安慰剂和治疗组每日一次50mg的收缩压最小二乘平均差为-9.6 mm Hg(90%CI,-15.8至-3.4 mm Hg;P=0.01),每日100mg的收缩压为-7.8 mm Hg(90%CI,-14.1至-1.5 mm Hg;P=.04)。在没有抑制PRA的参与者中,每天一次服用100mg氯前列素可将收缩压降低11.4 mm Hg(SD,2.5 mm Hg),这与接受相同剂量的抑制PRA参与者的血压降低相似。六名参与者的血清钾水平升高至6.0 mmol/L以上,并随着剂量减少或药物停药而得到纠正。没有出现皮质醇不足的情况。
结论和相关性:在高血压未得到控制的个体中,与安慰剂相比,使用氯前列素能有效降低血压,这将需要进一步的验证性研究[2]。
|
| 动物实验 |
Study Procedures [2]
Participants underwent 2 to 4 weeks of prescreening, a 2-week placebo run-in period to ensure eligibility, and then randomization and treatment for 8 weeks. A final visit was conducted 2 to 4 weeks following completion of the double-blind treatment (eFigure 1 in Supplement 2). Cohort 1 participants were randomly assigned in a 1:1:1:1:1:1 ratio to placebo or 1 of 5 lorundrostat doses (12.5 mg, 50 mg, or 100 mg once daily or 12.5 mg or 25 mg twice daily). An independent data and safety monitoring board performed an interim analysis in January 2022, and randomization to the 2 lowest doses of lorundrostat (12.5 mg once daily and 12.5 mg twice daily) was discontinued due to lack of consistent meaningful reduction of BP. A second cohort randomized participants to placebo or 100 mg once daily of lorundrostat in a 1:6 ratio. An additional interim analysis was performed following the last participant visit from cohort 1 and full enrollment of cohort 2 to guide dose selection in future studies. Following randomization, all participants’ study visits were conducted at weeks 1 through 8. Efficacy assessment with AOBP (average of the last 2 of 5 unattended measurements using an automated oscillometric sphygmomanometer device after approximately 5 minutes of rest in a seated position) was measured weekly throughout the study. Twenty-four-hour ambulatory BP monitoring was measured at baseline and once again prior to the 8-week visit. Sample Size Calculation and Power [2] The study was designed to provide information regarding a safe and effective dose of lorundrostat for subsequent efficacy trials. The sample size was based on the point and interval estimation of the difference in means between each dose group and placebo in change from baseline at week 8 in systolic AOBP. A sample size of 30 participants per group was estimated to provide the half-width of the 2-sided 90% CI of 3.8 mm Hg with a common standard deviation assumed to be 9 mm Hg. This was considered to have adequate precision to guide the evaluation of systolic BP changes across different dose levels for selection of doses and regimens to be investigated in future studies. All analyses of cohort 2 of the study were exploratory in nature, and no formal sample size considerations were undertaken for this cohort. |
| 毒性/毒理 (Toxicokinetics/TK) |
Safety End Points [2]
No participant deaths occurred during the trial. Three serious adverse events occurred; only 1 was deemed treatment related. A participant randomized to 100 mg once daily of lorundrostat in cohort 2 had worsening of hyponatremia necessitating stopping the drug. A total of 110 participants (55%) experienced any adverse event during the trial (Table 3). Most adverse events were classified as mild by investigators. No adrenocortical insufficiency occurred during the trial. Cosyntropin stimulation testing in participants randomized to 100 mg once daily in both cohort 1 and cohort 2 was normal, with stimulated values of cortisol greater than 18 µg/dL in all individuals receiving cosyntropin (eFigure 4 in Supplement 2). Prespecified adverse events of special interest included 3 participants (2%) with hypotension. Mean serum potassium increases were similar across all lorundrostat doses, including increases of 0.25 mmol/L in the 50-mg and 100-mg once-daily groups. Six participants (3.6%) had serum potassium levels above 6.0 mmol/L during the trial (Table 3). No instances of hyperkalemia required intervention beyond discontinuation or reduction in the dose of lorundrostat. |
| 参考文献 | |
| 其他信息 |
Importance: Excess aldosterone production contributes to hypertension in both classical hyperaldosteronism and obesity-associated hypertension. Therapies that reduce aldosterone synthesis may lower blood pressure.
Objective: To compare the safety and efficacy of lorundrostat, an aldosterone synthase inhibitor, with placebo, and characterize dose-dependent safety and efficacy to inform dose selection in future trials.
Design, setting, and participants: Randomized, placebo-controlled, dose-ranging trial among adults with uncontrolled hypertension taking 2 or more antihypertensive medications. An initial cohort of 163 participants with suppressed plasma renin (plasma renin activity [PRA] ≤1.0 ng/mL/h) and elevated plasma aldosterone (≥1.0 ng/dL) were enrolled, with subsequent enrollment of 37 participants with PRA greater than 1.0 ng/mL/h.
Interventions: Participants were randomized to placebo or 1 of 5 dosages of lorundrostat in the initial cohort (12.5 mg, 50 mg, or 100 mg once daily or 12.5 mg or 25 mg twice daily). In the second cohort, participants were randomized in a 1:6 ratio to placebo or lorundrostat, 100 mg once daily.
Main outcomes and measures: The primary end point was change in automated office systolic blood pressure from baseline to study week 8.[2]
Lorundrostat at doses of 50 mg and 100 mg once daily decreased AOBP significantly more than placebo. Blood pressure reduction was particularly evident among participants with hypertension and concomitant obesity. Lorundrostat was well tolerated, and small expected increases in serum potassium and declines in eGFR suggest a favorable safety profile, particularly with a 50-mg once-daily dose. The trial results support further study of lorundrostat as a treatment for uncontrolled hypertension.[1] |
| 分子式 |
C24H33N7O2
|
|---|---|
| 分子量 |
451.564524412155
|
| 精确质量 |
451.269
|
| 元素分析 |
C, 63.84; H, 7.37; N, 21.71; O, 7.09
|
| CAS号 |
1820940-17-7
|
| PubChem CID |
126567187
|
| 外观&性状 |
Light yellow to yellow solid powder
|
| LogP |
1.5
|
| tPSA |
103
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
7
|
| 可旋转键数目(RBC) |
6
|
| 重原子数目 |
33
|
| 分子复杂度/Complexity |
639
|
| 定义原子立体中心数目 |
0
|
| SMILES |
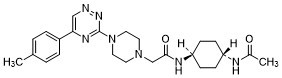
N1(CC(N[C@@H]2CC[C@@H](NC(C)=O)CC2)=O)CCN(C2=NC(C3=CC=C(C)C=C3)=CN=N2)CC1
|
| InChi Key |
YHGVDZULVMINCJ-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C24H33N7O2/c1-17-3-5-19(6-4-17)22-15-25-29-24(28-22)31-13-11-30(12-14-31)16-23(33)27-21-9-7-20(8-10-21)26-18(2)32/h3-6,15,20-21H,7-14,16H2,1-2H3,(H,26,32)(H,27,33)
|
| 化学名 |
N-(4-acetamidocyclohexyl)-2-[4-[5-(4-methylphenyl)-1,2,4-triazin-3-yl]piperazin-1-yl]acetamide
|
| 别名 |
Lorundrostat; 1820940-17-7; Lorundrostat [INN]; KA8W5LDS6Z; UNII-KA8W5LDS6Z; 1-Piperazineacetamide, N-(trans-4-(acetylamino)cyclohexyl)-4-(5-(4-methylphenyl)-1,2,4-triazin-3-yl)-; N-(trans-4-(Acetylamino)cyclohexyl)-4-(5-(4-methylphenyl)-1,2,4-triazin-3-yl)-1-piperazineacetamide; N-(trans-4-Acetamidocyclohexyl)-2-(4-(5-(4- methylphenyl)-1,2,4-triazin-3-yl)piperazin-1- yl)acetamide;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~33.33 mg/mL (~73.81 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (5.54 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (5.54 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.2145 mL | 11.0727 mL | 22.1455 mL | |
| 5 mM | 0.4429 mL | 2.2145 mL | 4.4291 mL | |
| 10 mM | 0.2215 mL | 1.1073 mL | 2.2145 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。