| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
MMP-3 (IC50 = 3 nM); MMP-1 (IC50 = 5 nM); MMP-2 (IC50 = 6 nM); MMP-14 (IC50 = 9 nM); MMP-7 (IC50 = 13 nM)
|
|---|---|
| 体外研究 (In Vitro) |
体外活性:Marimastat (100 nM) 显着抑制 U251、U87、GBM39 和 GBM43 肿瘤细胞中 MMP14 的表达。 Marimastat 特异性抑制神经胶质瘤细胞的生长,但对正常人星形胶质细胞 (NHA) 没有影响。 Marimastat 早期下调 Notch 靶基因的表达,例如 Hes1 和 Hes5。激酶测定:用 1 mM 乙酸 4-氨基苯汞在 37°C 下激活重组人 MMP2 1 小时。 1 μM 淬灭荧光 MMP 底物 (7-甲氧基香豆素-4-基)乙酰基-Pro-Leu-Gly-Leu-[3-(2,4-二硝基苯基)-L-2,3-二氨基丙酰基] 的裂解率-Ala-Arg-NH2 在 96 孔荧光板中于 37°C 下使用 320 nm 激发滤光片和 405 nm 100 mM Tris-HCl (pH 7.5)、100 mM NaCl、10 mM CaCl2、0.05% Brij 35 进行测量存在增加抑制剂浓度的情况下的发射过滤器。使用 GraphPad Prism 5.0 软件完成曲线拟合和 IC50 计算。细胞测定:在源自人神经胶质瘤细胞系 U251 和 GaMG 的肿瘤球体与 RBA 的共培养中,marimastat 在 10 μM 浓度下强烈抑制肿瘤侵袭。 Marimastat (10 μM) 可显着降低细胞增殖 54%,并且在 50 μM 的浓度下可在 6 天内完全抑制细胞生长。此外,marimastat (10 μM) 可使 U251 球体生长减少 65%。
|
| 体内研究 (In Vivo) |
在原位口腔鳞状细胞癌种植模型中,通过渗透泵给予马马司他(150 mg/kg/天,po)显着抑制颈部淋巴结转移和MMP-2的激活,并且比对照组具有显着更好的生存率。 Marimastat 降低多囊人和大鼠胆管细胞的 MMP 过度活跃,并阻止 PCK 胆管细胞的囊性扩张。用马马司他长期治疗 8 周大的 PCK 大鼠可抑制肝囊肿发生和纤维化。
|
| 酶活实验 |
在 37°C 下,用 1 mM 4-氨基苯汞乙酸盐激活重组人 MMP2 1 小时。测量了猝灭荧光 MMP 底物 1-甲氧基香豆素-4-基的裂解率。 Gly-Leu-乙酰基-Pro-Leu-[3-(2,4-二硝基苯基)-L-2,3-二氨基丙酰基] 在 37°C 下,在 96 孔荧光测定板中进行 Ala-Arg-NH2 的测量。在抑制剂浓度不断增加的情况下,使用 320 nm 激发滤光片和 405 nm 发射滤光片对 100 mM Tris-HCl (pH 7.5)、100 mM NaCl、10 mM CaCl2 和 0.05% Brij 35 进行测试。 GraphPad Prism 5.0软件用于IC50计算和曲线拟合。
|
| 细胞实验 |
在人胶质瘤细胞系 U251 和 GaMG 与浓度为 10 μM 的 RBA 的肿瘤球体共培养物中,Marimastat 显着减弱肿瘤侵袭。 Marimastat (10 μM) 在 50 μM 浓度下可在六天内完全抑制细胞生长,并显着降低 54% 的细胞增殖。此外,marimastat (10 μM) 65% 抑制 U251 球体的生长。
|
| 动物实验 |
Using a trochar needle, 2 mm2 of established SCC-1 tissue is subcutaneously injected into the flanks of three-month-old female naked mice. The tumors are treated when they reach a diameter of 5–6 mm. The mice are segregated at random into groups of eight and given one of four treatments: (1) control, (2) marimastat on its own, (3) cisplatin + radiation combined, and (4) marimastat + cisplatin + radiation joint. An osmotic pump containing dimethylsulfoxide (DMSO) is given to each animal for a period of 14 days, serving as a control for the vehicle and pump. Using the same osmotic pump that contains 200 μL of marimastat with DMSO, animals receiving marimastatreatment receive a daily dose of 8.7 mg/kg ten days after treatment starts. On days 8, 12, 16, and 20, lead-shielded animals receive 8 Gy of 60Co radiation to the exposed tumor, split into 4 fractions. Because 7.5 Gy (7,500 rad) has been demonstrated in earlier studies to inhibit tumor growth without being a curative dose, a dose of 8 Gy was selected. Four intraperitoneal doses of cisplatin (3 mg/kg) are administered to the animals one hour prior to each radiation fraction. For 32 days, tumors are measured every two weeks. Using mouse weight, potential treatment toxicity is tracked. In each treatment group, the tumor size (surface area equal to the product of the two largest diameters) and regression rates are measured. Tumors are removed for immunohistochemistry after 32 days. Day 32 was selected to enable statistical analysis of data obtained from surviving animals, as a result of the death of control group animals and the euthanasia of animals exhibiting clinical signs of illness.
|
| 参考文献 | |
| 其他信息 |
Marimastat is a secondary carboxamide resulting from the foraml condensation of the carboxy group of (2R)-2-[(1S)-1-hydroxy-2-(hydroxyamino)-2-oxoethyl]-4-methylpentanoic acid with the alpha-amino group of N,3-dimethyl-L-valinamide. It has a role as an antineoplastic agent and a matrix metalloproteinase inhibitor. It is a secondary carboxamide and a hydroxamic acid.
Used in the treatment of cancer, Marmiastat is an angiogenesis and metastasis inhibitor. As an angiogenesis inhibitor it limits the growth and production of blood vessels. As an antimetatstatic agent it prevents malignant cells from breaching the basement membranes. Marimastat is an orally-active synthetic hydroxamate with potential antineoplastic activity. Marimastat covalently binds to the zinc(II) ion in the active site of matrix metalloproteinases (MMPs), thereby inhibiting the action of MMPs, inducing extracellular matrix degradation, and inhibiting angiogenesis, tumor growth and invasion, and metastasis. This agent may also inhibit tumor necrosis factor-alpha converting enzyme (TACE), an enzyme involved in tumor necrosis factor alpha (TNF-alpha) production that may play a role in some malignancies as well as in the development of arthritis and sepsis. (NCI04) Drug Indication For the treatment of various cancers Mechanism of Action Marimastat is a broad spectrum matrix metalloprotease inhibitor. It mimics the peptide structure of natural MMP substrates and binds to matrix metalloproteases, thereby preventing the degradation of the basement membrane by these proteases. This antiprotease action prevents the migration of endothelial cells needed to form new blood vessels. Inhibition of MMPs also prevents the entry and exit of tumor cells into existing blood cells, thereby preventing metastasis. Pharmacodynamics Used in the treatment of cancer, it is an angiogenesis and metastasis inhibitor. As an angiogenesis inhibitor it limits the growth and production of blood vessels. As an antimetatstatic agent it prevents malignant cells from breaching the basement membranes. |
| 分子式 |
C15H29N3O5
|
|
|---|---|---|
| 分子量 |
331.41
|
|
| 精确质量 |
331.21
|
|
| 元素分析 |
C, 54.36; H, 8.82; N, 12.68; O, 24.14
|
|
| CAS号 |
154039-60-8
|
|
| 相关CAS号 |
|
|
| PubChem CID |
119031
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 密度 |
1.1±0.1 g/cm3
|
|
| 熔点 |
148℃
|
|
| 折射率 |
1.499
|
|
| LogP |
-0.16
|
|
| tPSA |
127.76
|
|
| 氢键供体(HBD)数目 |
5
|
|
| 氢键受体(HBA)数目 |
5
|
|
| 可旋转键数目(RBC) |
8
|
|
| 重原子数目 |
23
|
|
| 分子复杂度/Complexity |
431
|
|
| 定义原子立体中心数目 |
3
|
|
| SMILES |
O([H])[C@]([H])(C(N([H])O[H])=O)[C@]([H])(C(N([H])[C@]([H])(C(N([H])C([H])([H])[H])=O)C(C([H])([H])[H])(C([H])([H])[H])C([H])([H])[H])=O)C([H])([H])C([H])(C([H])([H])[H])C([H])([H])[H]
|
|
| InChi Key |
OCSMOTCMPXTDND-OUAUKWLOSA-N
|
|
| InChi Code |
InChI=1S/C15H29N3O5/c1-8(2)7-9(10(19)13(21)18-23)12(20)17-11(14(22)16-6)15(3,4)5/h8-11,19,23H,7H2,1-6H3,(H,16,22)(H,17,20)(H,18,21)/t9-,10+,11-/m1/s1
|
|
| 化学名 |
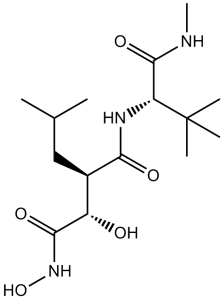
(2R,3S)-N-[(2S)-3,3-dimethyl-1-(methylamino)-1-oxobutan-2-yl]-N',3-dihydroxy-2-(2-methylpropyl)butanediamide
|
|
| 别名 |
BB 2516; TA-2516; Marimastat; BB-2516; BB2516; TA2516; TA 2516
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (7.54 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (7.54 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (7.54 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 50% DMSO+PBS: 30mg/mL 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.0174 mL | 15.0871 mL | 30.1741 mL | |
| 5 mM | 0.6035 mL | 3.0174 mL | 6.0348 mL | |
| 10 mM | 0.3017 mL | 1.5087 mL | 3.0174 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT00003011 | Completed | Drug: marimastat Drug: Placebo |
Lung Cancer | NCIC Clinical Trials Group | January 31, 1997 | Phase 3 |
| NCT00002911 | Completed | Drug: marimastat | Lung Cancer | ILEX Oncology Services, Incorporated |
December 1996 | Phase 3 |
| NCT00003010 | Completed | Drug: marimastat | Breast Cancer | Eastern Cooperative Oncology Group |
December 2, 1997 | Phase 3 |
| NCT00261391 | Completed | Drug: Marimastat | Vascular Anomalies | Boston Children's Hospital | October 2000 | Phase 1 |