| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| 10g | |||
| Other Sizes |
| 靶点 |
endogenous purines
Hypoxanthine-guanine phosphoribosyltransferase (HGPRT; but required for activation to 6-thioguanosine monophosphate) [1] - Thiopurine S-methyltransferase (TPMT; Ki=15 μM, substrate for methylation and inactivation) [1] - Orphan nuclear receptor NR4A3 (mediates glucose transport enhancement) [2] - DNA and RNA synthesis (inhibition via incorporation of thiopurine nucleotides; EC50 for leukemic cell lines: 0.5-5 μM) [1] |
|---|---|
| 体外研究 (In Vitro) |
巯嘌呤广泛用于治疗恶性肿瘤、风湿性疾病、皮肤病、炎症性肠病和实体器官移植排斥反应。巯嘌呤通过抑制磷酸核糖焦磷酸酰胺转移酶(PRPP 酰胺转移酶)来抑制嘌呤核苷酸的合成和代谢。 PRPP酰胺转移酶是嘌呤合成的限速酶。它改变 RNA 和 DNA 的合成和功能。巯嘌呤干扰核苷酸互变和糖蛋白合成。
72小时暴露后,对人急性淋巴细胞白血病(ALL)细胞系CCRF-CEM具有抗增殖活性,IC50为1.2 μM;诱导S期细胞周期阻滞,2 μM浓度下通过6-硫代鸟嘌呤核苷酸掺入DNA抑制DNA合成75%[1] - 增强L6大鼠骨骼肌细胞的葡萄糖转运活性;100 μM处理24小时,2-脱氧葡萄糖摄取较对照组增加60%;该效应可被NR4A3 siRNA部分阻断,提示依赖NR4A3的机制[2] - 诱导大鼠胎儿神经前体细胞(NPCs)的细胞周期阻滞(G1/S期)和凋亡;50 μM处理48小时,细胞活力降至45%,caspase-3活性升高2.5倍;TUNEL染色检测到凋亡细胞[3] - 抑制人HeLa细胞的RNA合成;3 μM 巯基嘌呤(6-MP)处理24小时,[3H]-尿苷掺入量减少60%[1] |
| 体内研究 (In Vivo) |
在6-巯基嘌呤水合物(6-MP)治疗组的胎儿端脑中,S期细胞群在治疗后36和48小时增加,并在治疗后72小时恢复到对照水平。 G2/M期细胞群在24小时开始增加,36小时达到峰值,48小时减少,最后在72小时恢复到对照水平。另一方面,亚G1期细胞群(凋亡细胞)在36小时开始增加,在48小时达到峰值,然后在72小时减少。
抑制裸鼠CCRF-CEM ALL异种移植瘤生长;每日口服50 mg/kg,持续14天,肿瘤生长抑制率(TGI)达65%(相较于溶媒对照组)[1] - 对妊娠母鼠给药后导致胎鼠神经毒性;妊娠第14天腹腔注射(i.p.)20 mg/kg,胎鼠脑内神经前体细胞数量减少50%,室管膜区凋亡细胞增加3倍[3] - 改变SD大鼠的糖代谢;每日口服30 mg/kg,持续7天,骨骼肌葡萄糖摄取增加45%,肌肉组织中NR4A3 mRNA表达上调2倍[2] |
| 酶活实验 |
L6 肌管在 DMSO 对照或 6-巯基嘌呤水合物 (6-MP) 中孵育 24 小时,最后 3 小时在无血清 DMEM 中进行处理。然后在存在或不存在 100 nM 胰岛素的情况下在 37°C 下再孵育 60 分钟。随后,收集 50 μg 蛋白质裂解物,进行 SDS-PAGE,然后使用一抗在 4°C 下进行一整夜的免疫印迹。使用 Image J 软件,对扫描胶片进行光密度分析,最终量化蛋白质 [2]。
采用纯化的人重组酶测定TPMT活性;将酶与5-50 μM 巯基嘌呤(6-MP)、S-腺苷甲硫氨酸(甲基供体)和Tris-HCl缓冲液(pH 7.5)在37°C下孵育30分钟;通过HPLC定量甲基化的巯基嘌呤(6-MP)代谢产物,以确定抑制效率并计算Ki值[1] - 采用纯化的大鼠肝脏HGPRT评估巯基嘌呤(6-MP)的HGPRT介导激活;将酶与1-20 μM 巯基嘌呤(6-MP)、磷酸核糖焦磷酸(PRPP,底物)和MgCl2在37°C下孵育60分钟;通过HPLC测定6-硫代鸟苷一磷酸的生成量[1] |
| 细胞实验 |
细胞活力测定用于量化细胞活力。将 10,000 个 L6 骨骼肌细胞接种到 96 孔板的每孔中,7 天后,细胞分化为肌管。测定前,用不同剂量的 6-巯基嘌呤水合物 (6-MP) 处理细胞 24 小时。室温平衡 30 分钟后,向每孔中添加 50 μL Cell Titer-Glo 试剂,并将板在定轨摇床上混合 12 分钟以分析细胞的活力。光度计用于测量光度[2]。
在96孔板中接种CCRF-CEM ALL细胞,每孔4×103个;贴壁24小时后,用0.1-10 μM 巯基嘌呤(6-MP)处理72小时;采用MTT法测定细胞活力,[3H]-胸腺嘧啶掺入法分析DNA合成,流式细胞术检测细胞周期分布[1] - 在24孔板中培养L6骨骼肌细胞;7天内分化为肌管;用25-200 μM 巯基嘌呤(6-MP)处理24小时;药物处理前48小时转染NR4A3 siRNA或 scramble siRNA;采用放射性测定法检测2-脱氧葡萄糖摄取,RT-PCR分析NR4A3 mRNA表达[2] - 分离大鼠胎儿神经前体细胞(E14),在6孔板中接种每孔1×105个;用10-100 μM 巯基嘌呤(6-MP)处理48小时;台盼蓝排斥法评估细胞活力,比色法测定caspase-3活性,TUNEL染色检测凋亡[3] |
| 动物实验 |
In this study, pregnant rats that are about thirteen weeks old are employed. The animals are kept in separate wire-mesh cages in an air-conditioned room with constant temperature and humidity levels (23±3°C and 50±20%, respectively), 10 cycles of ventilation (lights on for 12 hours and dark for 12 hours), and free access to pelleted food and water. In the experiment, three dams are each sacrificed by exsanguination from the abdominal aorta under ether anesthesia at 12, 24, 36, 48, and 72 hours after fifteen pregnant rats receive an intraperitoneal injection of 50 mg/kg 6-Mercaptopurine hydrate (6-MP) on E13. Each dam's fetuses are removed via Caesarean section. Three dams are sacrificed at each of the same time points, and fifteen pregnant rats are injected intraperitoneally (i.p.) with a 2.0% methylcellulose solution in distilled water as controls at E13[3].
Nude mice (6-8 weeks old) were implanted subcutaneously with 3×106 CCRF-CEM ALL cells; when tumors reached 100 mm3, Mercaptopurine (6-MP) was suspended in 0.5% carboxymethylcellulose sodium and administered orally at 50 mg/kg daily for 14 days; control mice received vehicle alone; tumor volume was measured every 3 days, and TGI was calculated [1] - Pregnant Sprague-Dawley rats (gestational day 12) were randomized into control and treatment groups; the treatment group received i.p. injection of 20 mg/kg Mercaptopurine (6-MP) (dissolved in normal saline) on gestational day 14; fetal brains were harvested on gestational day 18 for neural progenitor cell counting and TUNEL staining [3] - Adult Sprague-Dawley rats were given oral gavage of 30 mg/kg Mercaptopurine (6-MP) (suspended in 0.5% carboxymethylcellulose sodium) daily for 7 days; skeletal muscle tissue was collected after sacrifice; glucose uptake was measured ex vivo, and NR4A3 mRNA expression was analyzed by RT-PCR [2] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Clinical studies have shown that the absorption of an oral dose of mercaptopurine in humans is incomplete and variable, averaging approximately 50% of the administered dose. The factors influencing absorption are unknown. The volume of distribution exceeded that of the total body water. /MILK/ It is not known whether mercaptopurine is distributed into milk. Mercaptopurine and its metabolites are distributed throughout total body water. The volume of distribution of mercaptopurine usually exceeds total body water content. Although the drug reportedly crosses the blood-brain barrier, CSF concentrations are not sufficient for the treatment of meningeal leukemia. Mercaptopurine is excreted in urine as unchanged drug and metabolites. In one study in adults with normal renal function, about 11% of an oral dose was recovered in the urine within 6 hours. The immunosuppressant azathioprine is increasingly being used in pregnancy. The human placenta is considered a relative barrier to the major metabolite, 6-mercaptopurine (6-MP), and likely explains the lack of proven teratogenicity in humans. The aim of this study was to determine how the human placenta restricts 6-MP transfer using the human placental perfusion model. After addition of 50 ng/mL (n=4) and 500 ng/mL (n=3) 6-MP into the maternal circulation, there was a biphasic decline in its concentration and a delay in fetal circulation appearance. Under equilibrative conditions, the fetal-to-maternal concentration ratio was >1.0 as a result of ion trapping. Binding to placental tissue and maternal pharmacokinetic parameters are the main factors that restrict placental transfer of 6-MP. Active transport is unlikely to play a significant role and drug interactions involving, or polymorphisms in, placental drug efflux transporters are not likely to put the fetus at risk of higher 6-MP exposure. For more Absorption, Distribution and Excretion (Complete) data for Mercaptopurine (9 total), please visit the HSDB record page. Metabolism / Metabolites Hepatic. Degradation primarily by xanthine oxidase. The catabolism of mercaptopurine and its metabolites is complex. In humans, after oral administration of 35 S-6-mercaptopurine, urine contains intact mercaptopurine, thiouric acid (formed by direct oxidation by xanthine oxidase, probably via 6-mercapto-8-hydroxypurine), and a number of 6-methylated thiopurines. The methylthiopurines yield appreciable amounts of inorganic sulfate. After oral administration of 35(S)-6-mercaptopurine, urine contains intact mercaptopurine, thiouric acid (formed by direct oxidation by xanthine oxidase, probably via 6-mercapto-8-hydroxypurine), and a number of 6-methylated thiopurines. Mercaptopurine is metabolized via 2 major pathways. Mercaptopurine is rapidly and extensively oxidized to 6-thiouric acid in the liver by the enzyme xanthine oxidase. Because xanthine oxidase is inhibited by allopurinol, concomitant use of this drug decreases the metabolism of mercaptopurine and its active metabolites and leads to toxicity. If allopurinol and mercaptopurine are used concomitantly, the dosage of mercaptopurine must be reduced to avoid toxicity. Another major catabolic pathway is thiol methylation of mercaptopurine to form the inactive metabolite methyl-6-MP. This reaction is catalyzed by the enzyme thiopurine S-methyltransferase (TPMT). Variability in TPMT activity in patients because of a genetic polymorphism in the TPMT gene causes interindividual differences in the metabolism of mercaptopurine and resulting systemic exposure to the drug and its active metabolites. Dethiolation can also occur, with large portions of the sulfur being excreted as inorganic sulfate. ... In this study, we investigated the in vitro metabolism of 6-mercaptopurine (6MP) to 6-thiouric acid (6TUA) in pooled human liver cytosol. We discovered that 6MP is metabolized to 6TUA through sequential metabolism via the 6-thioxanthine (6TX) intermediate. The role of human AO and XO in the metabolism of 6MP was established using the specific inhibitors raloxifene and febuxostat. Both AO and XO were involved in the metabolism of the 6TX intermediate, whereas only XO was responsible for the conversion of 6TX to 6TUA. These findings were further confirmed using purified human AO and Escherichia coli lysate containing expressed recombinant human XO. Xanthine dehydrogenase (XDH), which belongs to the family of xanthine oxidoreductases and preferentially reduces nicotinamide adenine dinucleotide (NAD(+)), was shown to contribute to the overall production of the 6TX intermediate as well as the final product 6TUA in the presence of NAD(+) in human liver cytosol. In conclusion, we present evidence that three enzymes, AO, XO, and XDH, contribute to the production of 6TX intermediate, whereas only XO and XDH are involved in the conversion of 6TX to 6TUA in pooled HLC. The thiopurine antimetabolites, 6-mercaptopurine (6-MP) and 6-thioguanine (6-TG) are inactive pro-drugs that require intracellular metabolism for activation to cytotoxic metabolites. Thiopurine methyltransferase (TPMT) is one of the most important enzymes in this process metabolizing both 6-MP and 6-TG to different methylated metabolites including methylthioinosine monophosphate (meTIMP) and methylthioguanosine monophosphate (meTGMP), respectively, with different suggested pharmacological and cytotoxic properties. While meTIMP is a potent inhibitor of de novo purine synthesis (DNPS) and significantly contributes to the cytotoxic effects of 6-MP, meTGMP, does not add much to the effects of 6-TG, and the cytotoxicity of 6-TG seems to be more dependent on incorporation of thioguanine nucleotides (TGNs) into DNA rather than inhibition of DNPS. In order to investigate the role of TPMT in metabolism and thus, cytotoxic effects of 6-MP and 6-TG, we knocked down the expression of the gene encoding the TPMT enzyme using specifically designed small interference RNA (siRNA) in human MOLT4 leukemia cells. The knock-down was confirmed at RNA, protein, and enzyme function levels. Apoptosis was determined using annexin V and propidium iodide staining and FACS analysis. The results showed a 34% increase in sensitivity of MOLT4 cells to 1 uM 6-TG after treatment with TPMT-targeting siRNA, as compared to cells transfected with non-targeting siRNA, while the sensitivity of the cells toward 6-MP was not affected significantly by down-regulation of the TPMT gene. This differential contribution of the enzyme TPMT to the cytotoxicity of the two thiopurines is probably due to its role in formation of the meTIMP, the cytotoxic methylated metabolite of 6-MP, while in case of 6-TG methylation by TPMT substantially deactivates the drug. 6-Thiouric acid is the major metabolite of 6-mercaptopurine and is formed from this drug by the action of xanthine oxidase. Biological Half-Life Triphasic: 45 minutes, 2.5 hours, and 10 hours. Following IV administration of mercaptopurine (an IV preparation of the drug currently is not commercially available in the US), the elimination half-life of the drug is reportedly 21 minutes in pediatric patients and 47 minutes in adults. After an intravenous dose, the half-life of the drug in plasma is relatively short (about 50 minutes) due to uptake by cells, renal excretion, and rapid metabolic degradation. After iv administration of 6-mercaptopurine, the half-Iie for disappearance from the blood was about 9 min in rats and 14 min in mice. Oral bioavailability in humans is 10-20% due to first-pass metabolism in the liver [1] - Plasma half-life (t1/2) in humans is 1-2 hours; volume of distribution (Vd) is 0.5-1.0 L/kg [1] - Metabolized primarily by TPMT (methylation to inactive 6-methylmercaptopurine) and xanthine oxidase (XO, oxidation to 6-thiouric acid); 10% of the dose is excreted unchanged in urine [1] - Plasma protein binding rate is <10% in humans [1] |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
Mercaptopurine has been associated with several forms of hepatotoxicity. Patients receiving mercaptopurine for leukemia often have transient and asymptomatic rises in serum aminotransferase or alkaline phosphatase levels and a proportion of these patients develop jaundice, particularly when it is given in high doses. In case series of patients with autoimmune diseases (such as inflammatory bowel disease) treated with mercaptopurine, up to 30% developed serum aminotransferase elevations and these can be persistent as long as therapy is continued, resolving either with dose reduction or discontinuation. Liver biopsy usually demonstrates steatosis and centrolobular injury with scant inflammation. Mercaptopurine can also lead to a distinctive acute, clinically apparent liver injury that usually presents with fatigue and jaundice and a cholestatic or mixed pattern of serum enzyme elevations 1 to 6 months after starting therapy, but sometimes later, particularly following an increase in dose. Serum enzyme levels are often not very high, certainly not in the range that occurs with acute viral hepatiits. Rash, fever and eosinophilia are uncommon and autoantibodies are generally not found. Liver biopsy typically shows a mixed hepatocellular-cholestatic injury with cholestasis, focal hepatocellular necrosis, bile duct injury and variable amounts of inflammation. The injury is idiosyncratic and similar to the cholestatic hepatitis associated with azathioprine. The liver injury usually resolves upon stopping, but prolonged cholestasis has been reported and some cases have been fatal. In large case series and registries, mercaptopurine usually ranks among the top 20 causes of drug induced liver injury, and if combined with cases due to azathioprine [a prodrug of mercaptopurine] would rank among the top 10 more frequent causes. Chronic therapy with mercaptopurine and other thiopurines can lead to nodular regeneration and symptomatic portal hypertension. This chronic hepatotoxicity typically presents with fatigue and signs and symptoms of portal hypertension (ascites, varices), with mild liver enzyme abnormalities and minimal jaundice arising 6 months to many years after starting mercaptopurine. Liver biopsy shows nodular regenerative hyperplasia without significant fibrosis and varying amounts of sinusoidal dilation and central vein injury. This syndrome can progress to hepatic failure, particularly if mercaptopurine is continued, but gradual improvement on stopping therapy is typical. Rarely, the onset of this syndrome can be acute with abdominal pain and ascites in which situation liver biopsy usually shows sinusoidal dilation, central congestion and injury to sinusoidal endothelial cells suggestive of veno-occlusive disease, which is currently referred to as sinusoidal obstructive syndrome. Typically, serum aminotransferase levels and alkaline phosphatase levels are minimally elevated, even in the presence of hyperbilirubinemia and other manifestations of hepatic dysfunction and portal hypertension. Many cases present initially with unexplained thrombocytopenia, and progressive decreases in platelet counts may be the most sensitive marker for the development of the non-cirrhotic portal hypertension. Finally long-term therapy with mercaptopurine and other thiopurines has been implicated in leading to the development of malignancies, including hepatocellular carcinoma (HCC) and hepatosplenic T cell lymphoma (HSTCL). Both of these complications are rare but have been reported in several dozen case reports and small case series. In neither instance, has the role of thiopurine therapy in causing the malignacies been proven, and similar cases have been described in patients with autoimmune conditions or after solid organ transplantation who have not received thiopurines. Hepatocellular carcinoma typically arises after years of azathioprine or mercaptopurine therapy and in the absence of accompanying liver disease (although sometimes with focal hepatic glycogenosis). The HCC is most frequently found on an imaging study done of an unrelated condition. The prognosis is more favorable than that of HCC associated with cirrhosis. Hepatosplenic T cell lymphoma has been reported largely among young men with inflammatory bowel disease and long term immunosuppression with a thiopurine with or without anti-tumor necrosis factor therapy. The typical presentation is with fatigue, fever, hepatosplenomegaly and pancytopenia. The diagnosis is made by bone marrow or liver biopsy showing marked infiltration with malignant T cells. HSTCL is poorly responsive to antineoplastic therapy and has a high mortality rate. Likelihood score: A (well known cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation In the treatment of conditions such as ulcerative colitis and Crohn's disease, most professional guidelines and other experts consider breastfeeding to be acceptable during mercaptopurine therapy.[1-9] Azathioprine is rapidly converted to mercaptopurine, so data from mothers taking azathioprine apply to mercaptopurine. No active metabolites of mercaptopurine were found in the blood of breastfed infants whose mothers were taking azathioprine and only poorly documented cases of mild, asymptomatic neutropenia and increased rates of infection have been reported occasionally. It might be desirable to monitor exclusively breastfed infants with a complete blood count with differential, and liver function tests if azathioprine is used during lactation, although some authors feel that such monitoring is unnecessary.[10]. See the Azathioprine record for details. Mothers with decreased activity of the enzyme that detoxifies mercaptopurine metabolites may transmit higher levels of drug to their infants in breastmilk. It might be desirable to monitor exclusively breastfed infants with a complete blood count with differential, and liver function tests if mercaptopurine is used during lactation, although some authors feel that monitoring is unnecessary.[11] Avoiding breastfeeding for 4 hours after a dose should markedly decrease the dose received by the infant in breastmilk.[12] Most sources consider breastfeeding to be contraindicated during maternal antineoplastic drug therapy, although antimetabolites such as mercaptopurine appear to pose the least risk to breastfed infants.[13] After high-dose chemotherapy, it might be possible to breastfeed safely during intermittent therapy with an appropriate period of breastfeeding abstinence. Although no data are available to determine an appropriate period to withhold breastfeeding, the drug's terminal half-life suggests that withholding breastfeeding for 1 to 2 days may be sufficient. Chemotherapy may adversely affect the normal microbiome and chemical makeup of breastmilk.[14] ◉ Effects in Breastfed Infants In The Netherlands, 30 infants of mothers taking either azathioprine (n = 28) or mercaptopurine (n = 2) for inflammatory bowel disease during pregnancy and postpartum were followed at 1 to 6 years of age using a 43-item quality of life questionnaire. Of this cohort, 9 infants were breastfed for a mean of 7 months (range 3 to 13 months) No statistically significant differences were found between breastfed and formula-fed infants in any of the 12 domains of the survey.[19] In a multi-center study of women with inflammatory bowel disease in pregnancy (the PIANO registry), 102 women received a thiopurine (azathioprine or mercaptopurine) and another 67 received a thiopurine plus a biological agent (adalimumab, certolizumab, golimumab, infliximab, natalizumab, or ustekinumab) while breastfeeding their infants. Among those who received a thiopurine or combination therapy while breastfeeding, infant growth, development or infection rate was no different from 208 breastfed infants whose mothers received no treatment.[20] A national survey of gastroenterologists in Australia identified 21 infants who were breastfed by mothers taking a combination of allopurinol and a thiopurine (e.g. azathioprine, mercaptopurine) to treat inflammatory bowel disease. All had taken the combination during pregnancy also. Two postpartum infant deaths occurred, both at 3 months of age. One was a twin (premature birth-related) and the other from SIDS. The authors did not believe the deaths were medication related.[21] No information was provided on the extent of breastfeeding, drug dosages or the outcomes of the other infants. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Plasma protein binding averages 19% over the concentration range 10 to 50 µg/mL (a concentration only achieved by intravenous administration of mercaptopurine at doses exceeding 5 to 10 mg/kg). Myelosuppression (leukopenia, thrombocytopenia) is the major dose-limiting toxicity in humans; occurs at oral doses ≥1.5 mg/kg/day [1] - Hepatotoxicity (elevated serum transaminases) was observed in rats receiving oral doses of 40 mg/kg daily for 2 weeks [1] - Neurotoxicity in fetal rats: i.p. dose of 20 mg/kg on gestational day 14 caused reduced neural progenitor cell proliferation and increased apoptosis in fetal brain [3] - Drug-drug interaction: co-administration with allopurinol (XO inhibitor) increases plasma concentrations of Mercaptopurine (6-MP) by 2-3 fold, requiring dose reduction [1] - Cytotoxicity to normal human bone marrow stromal cells is low with CC50 >20 μM [1] |
| 参考文献 |
|
| 其他信息 |
Mercaptopurine can cause developmental toxicity according to state or federal government labeling requirements.
Purine-6-thiol is a thiol that is the tautomer of mercaptopurine. It has a role as an antineoplastic agent and an antimetabolite. It is a tautomer of a mercaptopurine. It derives from a hydride of a 7H-purine. An antimetabolite antineoplastic agent with immunosuppressant properties. It interferes with nucleic acid synthesis by inhibiting purine metabolism and is used, usually in combination with other drugs, in the treatment of or in remission maintenance programs for leukemia. Mercaptopurine anhydrous is a Nucleoside Metabolic Inhibitor. The mechanism of action of mercaptopurine anhydrous is as a Nucleic Acid Synthesis Inhibitor. Mercaptopurine (also referred to as 6-mercaptopurine or 6-MP) is a purine analogue that is effective both as an anticancer and an immunosuppressive agent, and is used to treat leukemia and autoimmune diseases as a corticosteroid-sparing agent. Mercaptopurine therapy is associated with a high rate of serum aminotransferase elevations which can be accompanied by jaundice. In addition, mercaptopurine has been linked to instances of clinically apparent acute liver injury and long term therapy to nodular regenerative hyperplasia. Mercaptopurine has been reported in Origanum dictamnus, Allium ampeloprasum, and other organisms with data available. Mercaptopurine is a thiopurine-derivative antimetabolite with antineoplastic and immunosuppressive activities. Produced through the metabolism of mercaptopurine by hypoxanthine-guanine phosphoribosyltransferase (HGPRT), mercaptopurine metabolites 6-thioguanosine-5'-phosphate (6-thioGMP) and 6-thioinosine monophosphate (T-IMP) inhibit nucleotide interconversions and de novo purine synthesis, thereby blocking the formation of purine nucleotides and inhibiting DNA synthesis. This agent is also incorporated into DNA in the form of deoxythioguanosine, which results in the disruption of DNA replication. In addition, mercaptopurine is converted to 6-methylmercaptopurine ribonucleoside (MMPR) by 6-thiopurine methyltransferase; MMPRs are also potent inhibitors of de novo purine synthesis. (NCI04) Mercaptopurine Anhydrous is the anhydrous form of mercaptopurine, a thiopurine-derivative antimetabolite with antineoplastic and immunosuppressive activities. Produced through the metabolism of mercaptopurine by hypoxanthine-guanine phosphoribosyltransferase (HGPRT), mercaptopurine metabolites 6-thioguanosine-5'-phosphate (6-thioGMP) and 6-thioinosine monophosphate (T-IMP) inhibit nucleotide interconversions and de novo purine synthesis, thereby blocking the formation of purine nucleotides and inhibiting DNA synthesis. This agent is also incorporated into DNA in the form of deoxythioguanosine, which results in the disruption of DNA replication. In addition, mercaptopurine is converted to 6-methylmercaptopurine ribonucleoside (MMPR) by 6-thiopurine methyltransferase; MMPRs are also potent inhibitors of de novo purine synthesis. An antimetabolite antineoplastic agent with immunosuppressant properties. It interferes with nucleic acid synthesis by inhibiting purine metabolism and is used, usually in combination with other drugs, in the treatment of or in remission maintenance programs for leukemia. Drug Indication For remission induction and maintenance therapy of acute lymphatic leukemia. FDA Label Xaluprine is indicated for the treatment of acute lymphoblastic leukaemia (ALL) in adults, adolescents and children. Treatment of acute lymphoblastic leukaemia Mechanism of Action Mercaptopurine competes with hypoxanthine and guanine for the enzyme hypoxanthine-guanine phosphoribosyltransferase (HGPRTase) and is itself converted to thioinosinic acid (TIMP). TIMP inhibits several reactions that involve inosinic acid (IMP), such as the conversion of IMP to xanthylic acid (XMP) and the conversion of IMP to adenylic acid (AMP) via adenylosuccinate (SAMP). Upon methylation, TIMP forms 6-methylthioinosinate (MTIMP) which inhibits glutamine-5-phosphoribosylpyrophosphate amidotransferase in addition to TIMP. Glutamine-5-phosphoribosylpyrophosphate amidotransferase is the first enzyme unique to the _de novo_ pathway for purine ribonucleotide synthesis. According to experimental findings using radiolabeled mercaptopurine, mercaptopurine may be recovered from the DNA in the form of deoxythioguanosine. In comparison, some mercaptopurine may be converted to nucleotide derivatives of 6-thioguanine (6-TG) via actions of inosinate (IMP) dehydrogenase and xanthylate (XMP) aminase that convert TIMP to thioguanylic acid (TGMP). The pathogenesis of several neurodegenerative diseases often involves the microglial activation and associated inflammatory processes. Activated microglia release pro-inflammatory factors that may be neurotoxic. 6-Mercaptopurine (6-MP) is a well-established immunosuppressive drug. Common understanding of their immunosuppressive properties is largely limited to peripheral immune cells. However, the effect of 6-MP in the central nervous system, especially in microglia in the context of neuroinflammation is, as yet, unclear. Tumor necrosis factor-alpha (TNF-a) is a key cytokine of the immune system that initiates and promotes neuroinflammation. The present study aimed to investigate the effect of 6-MP on TNF-a production by microglia to discern the molecular mechanisms of this modulation. Lipopolysaccharide (LPS) was used to induce an inflammatory response in cultured primary microglia or murine BV-2 microglial cells. Released TNF-a was measured by enzyme-linked immunosorbent assay (ELISA). Gene expression was determined by real-time reverse transcription polymerase chain reaction (RT-PCR). Signaling molecules were analyzed by western blotting, and activation of NF-kB was measured by ELISA-based DNA binding analysis and luciferase reporter assay. Chromatin immunoprecipitation (ChIP) analysis was performed to examine NF-kB p65 and coactivator p300 enrichments and histone modifications at the endogenous TNF-a promoter. Treatment of LPS-activated microglia with 6-MP significantly attenuated TNF-a production. In 6-MP pretreated microglia, LPS-induced MAPK signaling, I?B-a degradation, NF-kB p65 nuclear translocation, and in vitro p65 DNA binding activity were not impaired. However, 6-MP suppressed transactivation activity of NF-?B and TNF-a promoter by inhibiting phosphorylation and acetylation of p65 on Ser276 and Lys310, respectively. ChIP analyses revealed that 6-MP dampened LPS-induced histone H3 acetylation of chromatin surrounding the TNF-a promoter, ultimately leading to a decrease in p65/coactivator-mediated transcription of TNF-a gene. Furthermore, 6-MP enhanced orphan nuclear receptor Nur77 expression. Using RNA interference approach, we further demonstrated that Nur77 upregulation contribute to 6-MP-mediated inhibitory effect on TNF-a production. Additionally, 6-MP also impeded TNF-a mRNA translation through prevention of LPS-activated PI3K/Akt/mTOR signaling cascades. These results suggest that 6-MP might have a therapeutic potential in neuroinflammation-related neurodegenerative disorders through downregulation of microglia-mediated inflammatory processes. Mercaptopurine (6-MP) competes with hypoxanthine and guanine for the enzyme hyphoxanthine-guanine phosphoribosyltransferase (HGPRTase) and is itself converted to thioinosinic acid (TIMP). This intracellular nucleotide inhibits several reactions involving inosinic acid (IMP), including the conversion of IMP to xanthylic acid (XMP) and the conversion of IMP to adenylic acid (AMP) via adenylosuccinate (SAMP). In addition, 6-methylthioinosinate (MTIMP) is formed by the methylation of TIMP. Both TIMP and MTIMP have been reported to inhibit glutamine-5-phosphoribosylpyrophosphate amidotransferase, the first enzyme unique to the de novo pathway for purine ribonucleotide synthesis. Experiments indicate that radiolabeled mercaptopurine may be recovered from the DNA in the form of deoxythioguanosine. Some mercaptopurine is converted to nucleotide derivatives of 6-thioguanine (6-TG) by the sequential actions of inosinate (IMP) dehydrogenase and xanthylate (XMP) aminase, converting TIMP to thioguanylic acid (TGMP). Animal tumors that are resistant to mercaptopurine often have lost the ability to convert mercaptopurine to TIMP. However, it is clear that resistance to mercaptopurine may be acquired by other means as well, particularly in human leukemias. It is not known exactly which of any one or more of the biochemical effects of mercaptopurine and its metabolites are directly or predominantly responsible for cell death. Inflammatory bowel disease is characterized by chronic intestinal inflammation. Azathioprine and its metabolite 6-mercaptopurine (6-MP) are effective immunosuppressive drugs that are widely used in patients with inflammatory bowel disease. ... Azathioprine and 6-MP have been shown to affect small GTPase Rac1 in T cells and endothelial cells, whereas the effect on macrophages and gut epithelial cells is unknown. Macrophages (RAW cells) and gut epithelial cells (Caco-2 cells) were activated by cytokines and the effect on Rac1 signaling was assessed in the presence or absence of 6-MP. Rac1 is activated in macrophages and epithelial cells, and treatment with 6-MP resulted in Rac1 inhibition. In macrophages, interferon-gamma induced downstream signaling through c-Jun-N-terminal Kinase (JNK) resulting in inducible nitric oxide synthase (iNOS) expression. iNOS expression was reduced by 6-MP in a Rac1-dependent manner. In epithelial cells, 6-MP efficiently inhibited tumor necrosis factor-a-induced expression of the chemokines CCL2 and interleukin-8, although only interleukin-8 expression was inhibited in a Rac1-dependent manner. In addition, activation of the transcription factor STAT3 was suppressed in a Rac1-dependent fashion by 6-MP, resulting in reduced proliferation of the epithelial cells due to diminished cyclin D1 expression. These data demonstrate that 6-MP affects macrophages and gut epithelial cells beneficially, in addition to T cells and endothelial cells. Furthermore, mechanistic insight is provided to support development of Rac1-specific inhibitors for clinical use in inflammatory bowel disease. Mercaptopurine (6-MP) is a purine antimetabolite used in the treatment of hematological malignancies [1] - Its antitumor effect is mediated by intracellular activation to thiopurine nucleotides (6-thioguanosine triphosphate, 6-TGTP), which incorporate into DNA/RNA and inhibit nucleic acid synthesis [1] - TPMT genetic polymorphism affects drug metabolism: individuals with low TPMT activity are at increased risk of myelosuppression, requiring lower doses [1] - Approved by the FDA for the treatment of acute lymphoblastic leukemia (ALL) in children and adults, and for maintenance therapy of inflammatory bowel disease [1] - The glucose transport-enhancing effect in skeletal muscle suggests potential off-label applications in metabolic disorders [2] |
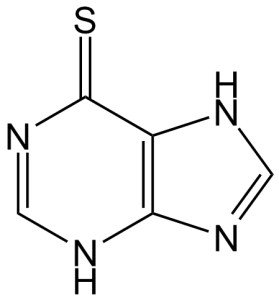
| 分子式 |
C5H4N4S
|
|
|---|---|---|
| 分子量 |
152.18
|
|
| 精确质量 |
152.015
|
|
| 元素分析 |
C, 39.46; H, 2.65; N, 36.82; S, 21.07
|
|
| CAS号 |
50-44-2
|
|
| 相关CAS号 |
6112-76-1 (hydrate); 50-44-2 (free)
|
|
| PubChem CID |
667490
|
|
| 外观&性状 |
Light yellow to yellow solid powder
|
|
| 密度 |
1.6±0.1 g/cm3
|
|
| 沸点 |
490.6±25.0 °C at 760 mmHg
|
|
| 熔点 |
241-244°C
|
|
| 闪点 |
250.5±23.2 °C
|
|
| 蒸汽压 |
0.0±1.2 mmHg at 25°C
|
|
| 折射率 |
1.820
|
|
| LogP |
-0.18
|
|
| tPSA |
89.45
|
|
| 氢键供体(HBD)数目 |
2
|
|
| 氢键受体(HBA)数目 |
2
|
|
| 可旋转键数目(RBC) |
0
|
|
| 重原子数目 |
10
|
|
| 分子复杂度/Complexity |
190
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
S=C1C2=C(N=C([H])N2[H])N([H])C([H])=N1
|
|
| InChi Key |
GLVAUDGFNGKCSF-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C5H4N4S/c10-5-3-4(7-1-6-3)8-2-9-5/h1-2H,(H2,6,7,8,9,10)
|
|
| 化学名 |
3,7-dihydropurine-6-thione
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 本产品在运输和储存过程中需避光。 |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (16.43 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (16.43 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (16.43 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 3.33 mg/mL (21.88 mM) in 50% PEG300 50% Saline (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 6.5712 mL | 32.8558 mL | 65.7117 mL | |
| 5 mM | 1.3142 mL | 6.5712 mL | 13.1423 mL | |
| 10 mM | 0.6571 mL | 3.2856 mL | 6.5712 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT05811845 | Recruiting | N/A | Acute Lymphoblastic Leukemia | IRCCS Burlo Garofolo | July 30, 2022 | N/A |
| NCT01503632 | Active Recruiting |
Behavioral: Behavioral Intervention Drug: Mercaptopurine |
Acute Lymphoblastic Leukemia | Children's Oncology Group | February 21, 2012 | Phase 3 |
| NCT02046694 | Completed | Drug: Allopurinol | Acute Lymphoblastic Leukemia (ALL) |
Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins |
January 6, 2014 | Early Phase 1 |
| NCT00648336 | Completed | Drug: Mercaptopurine 50 mg Drug: Purinethol® Tablets 50 mg |
Healthy | Mylan Pharmaceuticals Inc | November 2003 | Phase 1 |
| NCT01324336 | Completed | Drug: 6-Mercaptopurine | Acute Lymphoblastic Leukemia | Children's Mercy Hospital Kansas City |
July 2011 | N/A |
|
|
|
|