| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 2g |
|
||
| 5g |
|
||
| 10g |
|
||
| 50g |
|
||
| 100g |
|
||
| Other Sizes |
|
| 靶点 |
DNA synthesis
|
|---|---|
| 体外研究 (In Vitro) |
体外活性:甲硝唑在宿主或微生物细胞内代谢之前相对无活性。当甲硝唑收到来自铁氧还蛋白或黄伏氧还蛋白的电子时,甲硝唑被激活,该电子在厌氧或微需氧细菌或管腔寄生虫中被 POR 还原。甲硝唑通过形成蛋白质和 DNA 加合物来损害细胞。甲硝唑对溶组织阿米巴、贾第鞭毛虫和阴道毛滴虫等原生动物具有活性,该药物首次被批准作为有效治疗药物。甲硝唑对肠道厌氧菌群的活性已用于预防和治疗可能出现感染性并发症的克罗恩病患者。甲硝唑在厌氧菌相关感染中发挥着重要作用。甲硝唑对治疗厌氧性脑脓肿具有显着疗效。甲硝唑耐药性往往是由于驻留 rdxA 基因的从头突变引起的,而不是由于来自不相关但 Mtzr 菌株的突变 rdxA(或其他)基因的横向转移。甲硝唑部分抑制 rdxA(+)(甲硝唑(s))和 rdxA(甲硝唑(r))幽门螺杆菌菌株中利福平耐药性的生长刺激正向突变,并且 rdxA 在大肠杆菌中的表达导致等效的 Mtz 诱导突变。甲硝唑在纯人类杆菌的生长培养物中导致细胞凋亡样特征,包括程序性细胞死亡(PCD)的关键形态和生化特征,即。核浓缩和核内 DNA 缺口、细胞质体积减少、磷脂酰丝氨酸外化以及随着通透性增加而维持质膜完整性。
|
| 体内研究 (In Vivo) |
甲硝唑(135 mg/kg/d;口服;28 d)可穿透血脑屏障,长期给予大鼠时会表现出神经毒性[3]。
甲硝唑(1 g/L;口服;28 d)可穿透血脑屏障。 po;4 周)会导致骨骼肌萎缩,并改变与代谢调节和肌肉外周昼夜节律机制相关的基因表达[4]。 |
| 细胞实验 |
细胞系:芽囊菌 sp。细胞
浓度:0.1 μg/mL-0.01 mg/mL 孵育时间:12、24、48、60、72、84、96 小时 结果:细胞直径减小,作为凋亡的标志细胞,并导致细胞收缩。 |
| 动物实验 |
Sprague-Dawley (SD) rats (200-220 g)
135 mg/kg Oral gavage; once daily; 28 days |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
After the intravenous infusion of a 1.5g dose, peak concentration was reached within 1 hour and was peak level of 30-40 mg/L. When a multiple-dose regimen of 500mg three times a day administered intravenously, steady-state concentrations were achieved within about 3 days and peak concentration was measured at 26 mg/L. When administered orally in the tablet form, metronidazole is absorbed entirely absorbed, showing a bioavailability of greater than 90%. One resource indicates that Cmax after a single oral dose of 500mg metronidazole ranges from 8 to 13 mg/L, with a Tmax of 25 minutes to 4 hours. The AUC following a single 500mg oral dose of metronidazole was 122 ± 10.3 mg/L • h. A note on the absorption of topical preparations Insignificant percutaneous absorption of metronidazole occurs after the application of 1% metronidazole cream topically. Healthy volunteers applied one 100 mg dose of 14C-labelled metronidazole 2% cream to unbroken skin. After 12 hours, metronidazole was not detected in the plasma. Approximately 0.1% to 1% of the administered metronidazole was measured in the urine and feces. Metronidazole and metabolites are 60 to 80% eliminated in the urine, and 6-15% excreted in the feces. Metronidazole is widely distributed throughout the body and various body fluids. They include the bile, saliva, breastmilk, cerebrospinal fluid, and the placenta. Steady-state volume distribution of metronidazole in adults ranges from 0.51 to 1.1 L/kg. It attains 60 to 100% of plasma concentrations in various tissues, such as the central nervous system, however, is not measured in high concentrations in the placental tissue. Dose adjustments may be required in patients with hepatic impairment, as clearance is impaired in these patients. The clearance of metronidazole in the kidneys is estimated at 10 mL/min/1.73 m2. The total clearance from serum is about 2.1 to 6.4 L/h/kg. Well absorbed orally; bioavailability at least 80%. Distributed to saliva, bile, seminal fluid, breast milk, bone, liver and liver abscesses, lungs, and vaginal secretions; crosses the placenta and blood-brain barrier, also. At least 80% of an oral dose of metronidazole is absorbed from the GI tract. Following oral administration of a single 250-mg, 500-mg, or 2-g dose of metronidazole as immediate-release (conventional) preparations in healthy, fasting adults, peak plasma concentrations of unchanged drug and active metabolites are attained within 1-3 hours and average 4.6-6.5 ug/mL, 11.5-13 ug/mL, and 30-45 ug/mL, respectively. When a single 750-mg dose of metronidazole is administered as two 375-mg capsules or three 250-mg conventional tablets in healthy, fasting adult women, average peak plasma concentrations of unchanged drug and active metabolites of 20.4-21.4 ug/mL are attained in an average of 1.4-1.6 hours; metronidazole capsules and conventional tablets are bioequivalent at a single dose of 750 mg. The rate of absorption and peak plasma concentrations of metronidazole are decreased when conventional tablets or capsules of the drug are administered with food; however, the total amount of drug absorbed is not affected. Following oral administration of metronidazole 750 mg once daily as the extended-release tablet for 7 consecutive days in healthy, adult women, steady-state peak plasma concentrations average 12.5 mcg/mL and are attained an average of 6.8 hours after the dose when the drug is given under fasting conditions; when the drug is given at the same dosage under nonfasting conditions, steady-state peak plasma concentrations average 19.4 mcg/mL and are attained an average of 4.6 hours after the dose. Administration of metronidazole extended-release tablets with food increases the rate of absorption and peak plasma concentrations of the drug. According to the manufacturer, metronidazole extended-release and conventional tablets are bioequivalent at a dose of 750 mg given under fasting conditions. For more Absorption, Distribution and Excretion (Complete) data for METRONIDAZOLE (12 total), please visit the HSDB record page. Metabolism / Metabolites Metronidazole undergoes hepatic metabolism via hydroxylation, oxidation, and glucuronidation. The metabolism of metronidazole yields 5 metabolites. The hydroxy metabolite, 1-(2-hydroxy-ethyl)-2-hydroxy methyl-5-nitroimidazole, is considered the major active metabolite. Unchanged metronidazole is found in the plasma along with small amounts of its 2- hydroxymethyl metabolite. Several metabolites of metronidazole are found in the urine. They are primarily a product of side-chain oxidation in addition to glucuronide conjugation. Only 20% of the dose found in the urine is accounted for by unchanged metronidazole. The two main oxidative metabolites of metronidazole are hydroxy and acetic acid metabolites. Approximately 30-60% of an oral or IV dose of metronidazole is metabolized in the liver by hydroxylation, side-chain oxidation, and glucuronide conjugation. The major metabolite, 2-hydroxy metronidazole, has some antibacterial and antiprotozoal activity. ... Four other nitro-group-containing metabolites have been identified, each derived from side-chain oxidation of ethyl and/or methyl group. They include 1-acetic acid-2-methyl-5-nitroimidazole and 1-(2-hydroxyethyl)-2-carboxylic acid-5-nitroimidazole salt. The liver is the main site of metabolism, and this accounts for over 50% of the systemic clearance of metronidazole. The 2 principal metabolites result from oxidation of side chains, a hydroxy derivative and an acid. The hydroxy metabolite has a longer half-life (about 12 hr) and nearly 50% of the antitrichomonal activity of metronidazole. Formation of glucuronides also is observed. Small quantities of reduced metabolites, including ring-cleavage products, are formed by the gut flora. The urine of some patients may be reddish-brown owing to the presence of unidentified pigments derived from the drug. Hepatic metabolism by hydroxylation, oxidation, and glucuronidation. Half Life: 6-8 hours Biological Half-Life The elimination half-life of metronidazole is 7.3 ± 1.0 after a single 500mg IV dose in healthy subjects. Another resource indicates that the elimination half-life for metronidazole ranges from 6 to 10 hours. The plasma half-life of metronidazole is reported to be 6-8 hours in adults with normal renal and hepatic function. In one study using radiolabeled metronidazole hydrochloride, the half-life of unchanged metronidazole averaged 7.7 hours and the half-life of total radioactivity averaged 11.9 hours. The plasma half-life of metronidazole is not affected by changes in renal function; however, the half-life may be prolonged in patients with impaired hepatic function. In one study in adults with alcoholic liver disease and impaired hepatic function, half-life of metronidazole averaged 18.3 hours (range: 10.3-29.5 hours). Half-life: Neonates 25-75 hours; Others: 6-8 hours, increases with hepatic impairment. The elimination half-life in dogs is 4.5hr, and in horses 1.5-3.3hr |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
Metronidazole is a prodrug. Unionized metronidazole is selective for anaerobic bacteria due to their ability to intracellularly reduce metronidazole to its active form. This reduced metronidazole then covalently binds to DNA, disrupt its helical structure, inhibiting bacterial nucleic acid synthesis and resulting in bacterial cell death. Toxicity Data LD50=500 mg/kg/day (orally in rat). Interactions It is recommended that metronidazole not be used concurrently with, or for at least 1 day following, ingestion of alcohol; accumulation of acetaldehyde by interference with the oxidation of alcohol may occur, resulting in disulfiram-like effects such as abdominal cramps, nausea, vomiting, headache, or flushing; in addition, modifications in the taste of alcoholic beverages have been reported during concurrent use. Effects may be potentiated when /coumarin- or indandione-derivative anticoagulants/ are used concurrently with metronidazole, because of inhibition of enzymatic metabolism of anticoagulants; periodic prothrombin time determinations may be required during therapy to determine if dosage adjustments of anticoagulants are necessary. Hepatic metabolism of metronidazole may be decreased when metronidazole and cimetidine are used concurrently, possibly resulting in delayed elimination and increased serum metronidazole concentrations; monitoring of serum concentrations as a guide to dosage is recommended since dosage adjustments of metronidazole may be necessary during and after cimetidine therapy. It is recommended that metronidazole not be used concurrently with, or for 2 weeks following, disulfiram in alcoholic patients; such use may result in confusion and psychotic reactions because of combined toxicity. For more Interactions (Complete) data for METRONIDAZOLE (12 total), please visit the HSDB record page. Non-Human Toxicity Values LD50 Albino Rat oral > 5 g/kg |
| 参考文献 | |
| 其他信息 |
Therapeutic Uses
Mesh Heading: Anti-infective agents, antiprotozoal agents, radiation-sensitizing agents MEDICATION (VET): Antiprotozoal (Trichomonas); antiamebic; antibacterial MEDICATION (VET): The success of metronidazole in treating human infections of giardiasis, vaginal and oral trichomoniasis, and hepatic and intestinal amoebiasis has lead to investigation of its potential use against certain protozoan diseases of domestic animals. These are principally bovine urogenital trichomoniasis and canine, feline, or primate intestinal giardiasis, trichomoniasis, amoebiasis, or Balantidium infection. ... Oral metronidazole (extended release formulation) is used in the treatment of bacterial vaginosis caused by Gardnerella vaginalis, Mobiluncus spp, mycoplasma hominis and anaerobes (peptostreptococcus spp and Bacteroides spp). /Included in US or Canadian product labeling/ For more Therapeutic Uses (Complete) data for METRONIDAZOLE (25 total), please visit the HSDB record page. Drug Warnings Metronidazole crosses the placenta and enters the fetal circulation rapidly. Adequate and well-controlled studies in humans have not been done. ... However, the use of metronidazole in the treatment of trichomoniasis is not recommended during the first trimester. If metronidazole is used during the second and the third trimesters for trichomoniasis it is recommended that its use be limited to those patients whose symptoms are not controlled by local palliative treatment. Also, the 1 day course of therapy should not be used since this results in higher maternal and fetal serum concentrations. No information is available on the relationship of age to the effects of metronidazole in geriatric patients. However, elderly patients are more likely to have an age-related decrease in hepatic function, which may require an adjustment in dosage in patients receiving metronidazole. Peripheral neuropathy, characterized by numbness, tingling, or paresthesia of an extremity, and convulsive seizures have been reported rarely with oral or IV metronidazole. Peripheral neuropathy is usually reversible if metronidazole is discontinued but may persist in patients who receive prolonged therapy or higher than recommended dosage of the drug. Dizziness, vertigo, incoordination, ataxia, confusion, irritability, depression, weakness, insomnia, headache, syncope, tinnitus, and hearing loss have also occurred with metronidazole. Headache occurred in 18% of nonpregnant women receiving oral metronidazole (administered as extended-release tablets) for bacterial vaginosis, and among those reporting headache, 10% described it as severe. Urethral burning or discomfort, dysuria, cystitis, polyuria, incontinence, a sense of pelvic pressure, dryness of the vagina or vulva, dyspareunia, and decreased libido have been reported with oral metronidazole. Urine may be dark or reddish-brown in color following oral or IV administration of metronidazole due to the presence of water-soluble pigments which result from metabolism of the drug. Vulvovaginal candidiasis (or yeast vaginitis) was reported in 15% of nonpregnant women receiving oral metronidazole (administered as extended-release tablets) and in 12% of those receiving clindamycin phosphate (2% clindamycin) vaginal cream in a comparative study for the treatment of bacterial vaginosis. Although a definite causal relationship to the drug has not been established, genital pruritus, dysmenorrhea, and urinary tract infection have been reported in 5, 3, and 2%, respectively, of nonpregnant women receiving oral metronidazole (administered as extended-release tablets) for the treatment of bacterial vaginosis. For more Drug Warnings (Complete) data for METRONIDAZOLE (18 total), please visit the HSDB record page. Pharmacodynamics Metronidazole treats amebiasis, trichomoniasis, and giardiasis, exerting both antibacterial and antiprotozoal activities. Metronidazole is an effective treatment for some anaerobic bacterial infections. Metronidazole has shown antibacterial activity against the majority of obligate anaerobes, however, during in vitro studies, it does not demonstrate significant action against facultative anaerobes or obligate aerobes. The nitro group reduction of metronidazole by anaerobic organisms is likely responsible for the drug's antimicrobial cytotoxic effects, causing DNA strand damage to microbes. A note on convulsions and neuropathy and carcinogenesis It is important to be aware of the risk of peripheral neuropathy and convulsions associated with metronidazole, especially at higher doses. If convulsions or numbness of an extremity occur, discontinue the drug immediately. Metronidazole has been found to be carcinogenic in mice and rats. The relevance to this effect in humans is unknown. It is advisable to only administer metronidazole when clinically necessary and only for its approved indications. |
| 分子式 |
C6H9N3O3
|
|
|---|---|---|
| 分子量 |
171.15
|
|
| 精确质量 |
171.064
|
|
| 元素分析 |
C, 42.10; H, 5.30; N, 24.55; O, 28.04
|
|
| CAS号 |
443-48-1
|
|
| 相关CAS号 |
1460293-84-8 (sodium); 13182-82-6 (acetate); 443-48-1 (free); 13182-89-3 (benzoate); 69198-10-3 (HCl); 443-48-1
|
|
| PubChem CID |
4173
|
|
| 外观&性状 |
White to light yellow crystalline powder
|
|
| 密度 |
1.5±0.1 g/cm3
|
|
| 沸点 |
405.4±25.0 °C at 760 mmHg
|
|
| 熔点 |
159-161 °C(lit.)
|
|
| 闪点 |
199.0±23.2 °C
|
|
| 蒸汽压 |
0.0±1.0 mmHg at 25°C
|
|
| 折射率 |
1.612
|
|
| LogP |
-0.01
|
|
| tPSA |
83.87
|
|
| 氢键供体(HBD)数目 |
1
|
|
| 氢键受体(HBA)数目 |
4
|
|
| 可旋转键数目(RBC) |
2
|
|
| 重原子数目 |
12
|
|
| 分子复杂度/Complexity |
170
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
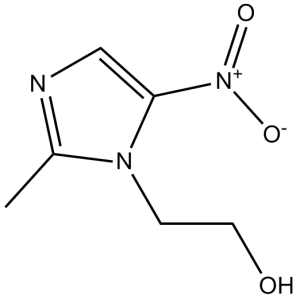
O([H])C([H])([H])C([H])([H])N1C(=C([H])N=C1C([H])([H])[H])[N+](=O)[O-]
|
|
| InChi Key |
VAOCPAMSLUNLGC-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C6H9N3O3/c1-5-7-4-6(9(11)12)8(5)2-3-10/h4,10H,2-3H2,1H3
|
|
| 化学名 |
2-(2-methyl-5-nitroimidazol-1-yl)ethanol
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 本产品在运输和储存过程中需避光。 |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (12.15 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (12.15 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (12.15 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 12.5 mg/mL (73.04 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶 (<60°C). 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 5.8428 mL | 29.2141 mL | 58.4283 mL | |
| 5 mM | 1.1686 mL | 5.8428 mL | 11.6857 mL | |
| 10 mM | 0.5843 mL | 2.9214 mL | 5.8428 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Standard Versus Prolonged Antibiotic Prophylaxis After Pancreatoduodenectomy (SPARROW)
CTID: NCT05784311
Phase: Phase 4 Status: Recruiting
Date: 2024-10-21
|