| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
PHD1 (IC50 = 480 nM); PHD2 (IC50 = 280 nM); PHD3 (IC50 =450 nM)[1]
|
||
|---|---|---|---|
| 体外研究 (In Vitro) |
对于 PHD1、PHD2 和 PHD3,BAY 85-3934 的平均 IC50 值分别为 480 nM、280 nM 和 450 nM。 HeLa 细胞只需暴露于 5 μM BAY 85-3934 20 分钟即可产生可检测水平的 HIF-1α。使用缺氧反应元件启动子作为对照,BAY 85-3934 在细胞报告基因测定中诱导萤火虫荧光素酶报告基因的表达,平均 (± SD) EC50 为 8.4±0.7 μM (n=4) [1] 。
|
||
| 体内研究 (In Vivo) |
当健康 Wistar 大鼠和食蟹猴口服 HIF-PH 抑制剂 BAY 85-3934 (Molidustat) 时,它可以稳定 HIF 并导致 EPO 的剂量依赖性产生。与 rhEPO 治疗相比,除了使 CKD 大鼠模型的高血压血压正常化外,莫度司他治疗还可有效控制肾功能受损大鼠的肾性贫血 [1]。
|
||
| 酶活实验 |
脯氨酰羟化酶测定[1]
脯氨酰羟化酶测定如前所述,稍作修改。生物素化HIF-1α556–574(生物素DLDLDLELMLAPYIPMDDDFQL)与白色96孔NeutrAvidin高结合能力板结合,该板用阻断剂酪蛋白预阻断,随后用1 mM生物素阻断。将固定的肽底物与适量的HIF-PH在含有20 mM Tris(PH 7.5)、5 mM KCl、1.5 mM MgCl2、20µM 2-酮戊二酸、10µM FeSO4、2 mM抗坏血酸、4%蛋白酶抑制剂(不含EDTA,罗氏诊断公司)的缓冲液中孵育,最终体积为100µl,加入或不加入适当浓度的测试化合物。反应时间为60分钟。为了停止反应,用洗涤缓冲液洗涤板三次。[1] 羟基化生物素-HHIF-1α556–574与Eu-VBC在100µl结合缓冲液(50 mM Tris[pH 7.5],120 mM NaCl)中在室温下孵育60分钟。用DELFIA洗涤缓冲液洗涤六次并加入100µl增强剂溶液后,通过Tecan无限M200平板读数器测量时间分辨荧光来确定结合的VBC的量。测量重复三次或更多次,结果以平均值±SEM表示。使用GraphPad Prism软件对数据集应用四参数逻辑方程进行曲线拟合后,确定IC50值。当需要调节游离Fe2+的浓度时,向反应缓冲液中补充适量的硫酸铁(II)铵((NH4)2Fe(SO4)2.6H2O,莫尔盐)。 |
||
| 细胞实验 |
细胞系、细胞培养基和萤光素酶报告测定[1]
A549和HeLa癌细胞系(美国典型培养物保藏中心)在DMEM/F-12中培养,Hep3B细胞在RPMI培养基中培养,两者均添加了抗生素、L-谷氨酰胺和10%胎牛血清。用HIF-RE2-luc HIF报告构建体(在pGL3中构建)稳定转染的A549细胞以2500个细胞/孔的密度接种在384孔板上,体积为25µl的完整细胞培养基中,并在测试前重新孵育16-24小时。以10µl的体积加入适当稀释的试验化合物,并在测量前将细胞重新孵育6小时。在加入细胞裂解/萤光素酶缓冲液后,在光度计中测定萤光素酶活性。通过STR DNA分型验证细胞系身份。 蛋白质印迹分析[1] 对于蛋白质印迹分析,细胞裂解物在4-12%SDS-聚丙烯酰胺梯度凝胶上分离。蛋白质被印迹到聚偏二氟乙烯(PVDF)膜上。使用HIF-1α特异性单克隆抗体以1∶250的稀释度检测HIF-1α蛋白。使用稀释度为1∶1000的HIF-2α特异性多克隆抗体检测HIF-2α蛋白。抗β-肌动蛋白抗体作为负载对照。根据制造商的说明,通过结合辣根过氧化物酶偶联的抗小鼠IgG抗体来观察抗体的结合,随后使用化学发光增强。Novex Sharp预染色蛋白标准品用作分子量标记。 |
||
| 动物实验 |
|
||
| 毒性/毒理 (Toxicokinetics/TK) |
Overall, 94.5% of patients experienced at least 1 TEAE during the study: 92.7% of patients in the molidustat group and 96.3% in the darbepoetin group (Table 2). The most commonly reported TEAEs were nasopharyngitis (34.1% and 40.2% in the molidustat and darbepoetin groups, respectively), worsening of CKD (18.3% and 9.8%, respectively), and diarrhea (8.5% and 12.2%, respectively) (Table 2). TEAEs leading to death were reported in 2 patients (2.4%) in the molidustat group and none in the darbepoetin group, and serious TEAEs were reported in 32.9% and 26.8% of patients, respectively. MACEs that occurred after the start of the study drug were reported in 3.7% of patients treated with molidustat and 1.2% of patients receiving darbepoetin (online suppl. Table 3). Additionally, 3.7% of patients in the molidustat group and 1.2% in the darbepoetin group developed diabetic retinopathy, and 3.7% in the molidustat group and 4.9% in the darbepoetin group developed neoplasms (benign, malignant, or unspecified) (online suppl. Table 4). The mean serum eGFR appeared to remain stable in the molidustat group (online suppl. Fig. 7). Subgroup analyses of TEAEs by age group (<65 and ≥65 years old) and by sex are presented in online supplementary Table 5. The proportion of serious TEAEs was similar between the 2 groups in female patients but higher for males in the molidustat group than in the darbepoetin group.[2]
|
||
| 参考文献 |
|
||
| 其他信息 |
Molidustat is under investigation in clinical trial NCT03350321 (A Study of Molidustat for Correction of Renal Anemia in Non-dialysis Subjects).
See also: Molidustat Sodium (active moiety of). |
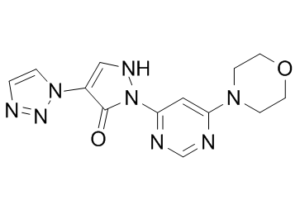
| 分子式 |
C13H14N8O2
|
|
|---|---|---|
| 分子量 |
314.3
|
|
| 精确质量 |
314.123
|
|
| 元素分析 |
C, 49.68; H, 4.49; N, 35.65; O, 10.18
|
|
| CAS号 |
1154028-82-6
|
|
| 相关CAS号 |
1375799-59-9 (Sodium);1154028-82-6;
|
|
| PubChem CID |
59603622
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 密度 |
1.7±0.1 g/cm3
|
|
| 沸点 |
589.2±60.0 °C at 760 mmHg
|
|
| 闪点 |
310.2±32.9 °C
|
|
| 蒸汽压 |
0.0±1.7 mmHg at 25°C
|
|
| 折射率 |
1.820
|
|
| LogP |
-1.77
|
|
| tPSA |
106.75
|
|
| 氢键供体(HBD)数目 |
1
|
|
| 氢键受体(HBA)数目 |
8
|
|
| 可旋转键数目(RBC) |
3
|
|
| 重原子数目 |
23
|
|
| 分子复杂度/Complexity |
481
|
|
| 定义原子立体中心数目 |
0
|
|
| InChi Key |
IJMBOKOTALXLKS-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C13H14N8O2/c22-13-10(20-2-1-16-18-20)8-17-21(13)12-7-11(14-9-15-12)19-3-5-23-6-4-19/h1-2,7-9,17H,3-6H2
|
|
| 化学名 |
2-(6-morpholin-4-ylpyrimidin-4-yl)-4-(triazol-1-yl)-1H-pyrazol-3-one
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 0.5 mg/mL (1.59 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 5.0 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 0.5 mg/mL (1.59 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 5.0 mg/mL 澄清 DMSO 储备液加入 900 μL 20% SBE-β-CD 生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 0.5 mg/mL (1.59 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 10 mg/mL (31.82 mM) in 0.5% CMC-Na/saline water (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.1817 mL | 15.9084 mL | 31.8167 mL | |
| 5 mM | 0.6363 mL | 3.1817 mL | 6.3633 mL | |
| 10 mM | 0.3182 mL | 1.5908 mL | 3.1817 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。