| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
| 靶点 |
Fluorescent dye for monitoring glucose uptake in living cells and tissues
|
|---|---|
| 体外研究 (In Vitro) |
在体外(步骤1-2)或体内(步骤3-7)进行MQAE染色,然后进行成像(步骤8)。
MQAE对培养细胞或组织切片的染色
该方案允许对切片上部70-120µm进行高质量染色,以便根据其形态识别不同类型的神经元(Marandi等人,2002;参见图1)。 1. 将MQAE溶解于小鼠标准生理盐水中至终浓度6mM。 2. 将培养的细胞或脑切片与该溶液在37℃下孵育10分钟,然后用无染料生理盐水冲洗10 - 15分钟。 |
| 体内研究 (In Vivo) |
In Vivo Staining of Neurons and Glia Using Multicell Bolus Loading [1]
这种方法允许在直径约200µm的球形体积内染色神经元和胶质细胞(见图2)。 3. 根据《使用多细胞丸加载的体内双光子钙成像》(Garaschuk and Konnerth 2010)对小鼠大脑进行手术 4. 使用Ultrafree-MC离心过滤器过滤新制备的移液染色液。 5. 用染色溶液填充类似于膜片移液器的移液管(填充的移液管的阻力为3-6 MΩ),并使用LN-Mini机械手将其放置在脑组织内所需深度的光学控制下。使用成像系统连续监测移液器位置。 6. 使用短暂(仅500毫秒)的弹射脉冲(弹射压力34.5千帕)将MQAE压射到大脑中。以1-3分钟的脉冲间隔重复喷射2 - 4次。 7. 在最后一次喷射脉冲后10分钟检查获得的染色质量。 这个短暂的等待时间是允许MQAE从细胞外空间冲洗出来所必需的(很可能是因为微循环)。与膜渗透钙指示染料相比,MQAE在细胞内不进行脱酯化;因此,无需额外的等待时间。 MQAE染色细胞的双光子成像 8. 进行双光子成像 在单光子激发下,MQAE的激发波长为320-400 nm,最大发射波长为460 nm (Verkman et al. 1989)。通过双光子成像,MQAE在~ 740-770 nm处被有效激发。也可以在更长的波长(高达800纳米)激发MQAE,但发射光的强度较低(Marandi et al. 2002)。利用我们的成像系统,在960 ~ 990 nm的激发波长处无法激发MQAE。 MQAE的胞内校正 用Cl -淬灭喹啉基Cl -指示剂的效率取决于溶剂的粘度和/或极性(Jayaraman和Verkman 2000),因此,与试管试验相比,在细胞内可能不同。Krapf et al.引入的校准方案可用于校准切片神经元中的Cl -水平(Krapf et al. 1988;Marandi et al. 2002). 9. 制备含有不同量Cl−的体外校准溶液(例如,0、10、20、30和40 mM)。分别加入三丁基氯化锡(10µM)和尼日利亚菌素(10µM) 这种处理将破坏跨细胞膜的Cl -梯度,并将确定细胞质Cl -浓度([Cl -]i)等于相应的校准溶液的浓度。 10. 依次应用体外校准液,测量细胞内稳态荧光水平。无Cl−溶液中的平均荧光水平定义为F0。将每个校准溶液的F值绘制为F0/F相对于相应的[Cl−]i(所谓的Stern-Volmer图)。回归线的斜率(Stern-Volmer常数KSV)是表观解离常数Kd的倒数。 在我们的校准实验中,MQAE在试管内的Kd为13 mM,在体外脑片神经元内的Kd为40 mM (KSV = 24.7 M-1) (Marandi et al. 2002)。在其他组织中,KSV值在3 - 26 M-1之间变化(Lau等,1994;Bevensee et al. 1997;Maglova et al. 1998;Eberhardson et al. 2000;Gilbert et al. 2007;Hille et al. 2009)。 故障排除 问题(步骤2和7):观察到染色不良。 解决方案:MQAE染色本身比较简单可靠。然而,它需要高质量的切片/体内制备。考虑以下内容: 1. 为了获得高质量的体内制剂,请遵循使用多细胞丸加载的体内双光子钙成像(Garaschuk and Konnerth 2010)中描述的建议。 2. 我们不建议一次交付大量的MQAE。看起来MQAE从细胞外间隙被冲洗的效果不如膜渗透钙指示染料,大量染料的积累导致模糊(低对比度-高亮度)染色。 |
| 细胞实验 |
本方案描述了一种使用基于喹啉的氯化物(Cl−)指示染料MQAE (N-[6-甲氧基喹啉]乙酰乙酯)对活细胞进行高分辨率氯化物成像的技术。应用于急性脑切片,MQAE提供了神经元细胞及其过程的高质量标记。在活麻醉小鼠中,使用多细胞丸加载程序标记皮质细胞。结合双光子显微镜,该方法可以在体内可视化神经元和星形胶质细胞的细胞体以及一些星形胶质细胞的过程,并允许人们监测几十个单个细胞内细胞内氯化物浓度的变化。[1]
材料 你们务必查阅相应的物质安全数据表和你们机构的环境健康与安全办公室,以正确处理本方案中使用的设备和危险材料 试剂 体外定标液 MQAE (体外染色) MQAE试管校准溶液 尼日利亚素(K+/H+离子载体) 移液染色液,新鲜配制(用于体内染色) 标本 培养小鼠细胞或脑切片(用于体外染色) 所需菌株小鼠(用于体内染色) 小鼠标准外用生理盐水(体外染色用) 手术和麻醉试剂,如《使用多细胞丸加载的体内双光子钙成像》(Garaschuk和Konnerth 2010)(用于体内染色)中所述。 氯化三丁基锡(Cl−/OH−反转运物) 设备 玻璃毛细血管(用于体内染色) 图像的设置 任何市售的双光子成像系统都可以使用。这样的系统可以从几个供应商处获得。我们目前使用的是一个定制的装置,该装置基于一个锁模Ti:蓝宝石激光器,具有自动色散补偿和一个激光扫描系统,耦合到一个垂直显微镜,并配备了一个60×, 1.0数值孔径(NA)的水浸物镜(Fluor 60x;尼康)。这种定制系统可以按照Majewska等人(2000)和Nikolenko和Yuste(2005)所描述的说明进行组装。我们在750-770 nm处激发MQAE,在400 - 720 nm处采集荧光。然后使用ImageJ程序(http://rsb.info.nih.gov/ij/)和基于labview的软件包对获取的图像进行背景校正和离线分析。 培养箱,预设到37°C(用于体外染色) LN-Mini机械手(用于体内染色) 移液器(用于体内染色) 压力应用系统(用于体内染色) 带有中央通道开口的记录室:由标准组织培养皿(直径35毫米;Garaschuk et al. 2006)。 手术和麻醉设备,描述在体内双光子钙成像使用多细胞丸加载(Garaschuk和Konnerth 2010)(用于体内染色) Ultrafree-MC离心滤芯,孔径0.45µm(体内染色) |
| 动物实验 |
Female mice (C57BL/6J, 5 weeks of age) were fed standard pellet food and water ad libitum. Airway ciliary cells were isolated from the lungs as previously described. Briefly, the mice were anesthetized by 3% isoflurane (inhalation) and then further anesthetized by an intraperitoneal injection (ip) of pentobarbital sodium (40 mg/kg) and heparinized (1000 U/kg) for 15 min. The mice were then sacrificed by a high-dose of pentobarbital sodium (100 mg/kg, ip) and the airway ciliary cells isolated by an elastase treatment.[4]
Measurement of MQAE fluorescence intensities [4] MQAE was dissolved in a mixture of acetonitrile and water (1:1; stock solution), and the stock solution (500 mM) was stored at − 20 °C. Isolated airway ciliary cells were incubated with 10 mM MQAE for 60 min at 37 °C. MQAE at 5 mM is widely used for intracellular loading in many cell types, but at this concentration the airway ciliary cells in our study were not sufficiently stained for MQAE within 60 min. We thus used a 10 mM MQAE to obtain sufficient staining to measure the MQAE fluorescence intensity in the airway ciliary cells. The same concentration (10 mM) was used to measure [Cl−]i in A6 cells. The MQAE-loaded cells were set on a coverslip pre-coated with Cell-Tak, which was set in a micro-perfusion chamber (20 µl) mounted on an inverted light microscope equipped with a confocal laser scanning system. MQAE was excited at 780 nm using a two-photon excitation laser system, and emission was at 510 nm. The normalized value of fluorescence intensity (Ft/F0) was calculated; the subscripts “0” or “t” indicate the time just before or just after the start of application of osmotic stress, respectively. The airway ciliary cells were observed in the optical sections (thickness 0.9 μm) using the confocal laser scanning microscope. The cell volume was measured by tracing the outline of a ciliary cell on the phase contrast image of each optical section, and the area (An µm2) was measured; “n” shows the number of optical sections. We also measured the MQAE fluorescence intensity (Fn) in the intracellular area of the cell in each optical section. The image analysis system reported Fn as intensity per micron2. The cell volume (V) was calculated by the sum of An in each section. We also calculated the total MQAE fluorescence intensity of the all cell areas by summing the total fluorescence intensity (An × Fn) in each section. The total MQAE fluorescence intensity in all cell areas indicates [Cl−]i, if the number of MQAE molecules does not change. We obtained 18–22 optical sections from each cell. The normalized value of cell volume (Vt/V0) was also calculated using the sum of An, whereby the subscripts “0” or “t” indicate the time just before or after the application of osmotic stress, respectively. We also measured the changes in MQAE fluorescence intensity (Ft/F0) in the selected local area of the selected cell using the identical focal plane throughout the experiment; the subscripts “0” or “t” indicate the time just before or after the osmotic stress, respectively. |
| 参考文献 |
|
| 其他信息 |
The importance of chloride channels for the cell is demonstrated by a number of serious human diseases that are due to mutations in chloride channels. The most well-known of these diseases is cystic fibrosis. Investigations into the mechanisms of the disease and possible treatments require the study of chloride fluxes at the level of individual cells. The present study compares two methods for studies of chloride transport: X-ray microanalysis and MQAE fluorescence with image analysis. As an experimental system, the cAMP-activated chloride channel in cultured respiratory epithelial cells was chosen. Both methods showed that stimulation with the cAMP-elevating agents forskolin and IBMX decreased the chloride content of the cells by about 20-27%. Inducing a driving force for chloride by replacing extracellular chloride by nitrate resulted in a chloride efflux that was significantly increased in the presence of forskolin and IBMX. This study shows that X-ray microanalysis and MQAE fluorescence are adequate and comparable methods for measuring cAMP-dependent chloride transport in individual cells.[2]
A novel fluorescent indicator, N-[ethoxycarbonylmethyl]-6-methoxy-quinolinium bromide (MQAE), was used to measure intracellular chloride concentration ([Cl-]i) in primary cultures of rat aortic smooth muscle cells (VSMC). The hydrolytic and fluorescent properties of the dye were characterized. The intracellular Stern-Volmer constant was calculated to be 25 M-1. Cl- efflux curves were characteristic of saturation-type kinetics, with an apparent Michaelis-Menten constant value of 11 +/- 4.8 (SD) mM, a maximum velocity of 0.038 +/- 0.021 mM/s, and a half time (t1/2) of 9.0 +/- 3.7 min. The average efflux rate in the first 10 min (0.023 +/- 0.004 mM/s) was reduced in the presence of either 130 microM 4,4'-diisothiocyanato-dihydrostilbene-2,2'-disulfonic acid (H2DIDS) (0.014 +/- 0.006, P = 0.02) or 40 microM furosemide (0.017 +/- 0.004, P = 0.04). Restoration of physiological extracellular chloride concentration ([Cl-]o) after zero Cl- resulted in net Cl- influx with a t1/2 of 3.6 +/- 1.0 min. The initial Cl- influx rate was reduced after exposure to furosemide, from 0.069 +/- 0.006 to 0.046 +/- 0.008 mM/s, P < 0.002, and was reduced after exposure to H2DIDS from 0.102 +/- 0.013 to 0.033 +/- 0.003 mM/s, P < 0.001. Furosemide reduced the steady-state [Cl-]i from 31.6 +/- 3.2 to 26.1 +/- 2.4 mM, P < 0.01, whereas H2DIDS had little effect on [Cl-]i. Our results demonstrate that MQAE can be used to measure [Cl-]i in primary cultures of VSMC.[3] Advantages and Limitations[1] MQAE provides easy and fast staining of neurons in vitro and in vivo with satisfactory fluorescence levels in cell bodies. In brain slices, it also allows one to image neuronal dendrites, whereas in vivo only glial processes can clearly be discerned. This discrepancy is most probably caused by slow/incomplete wash-out of the dye from the extracellular space under the in vivo conditions. Compared with other Cl− indicators, the advantages of MQAE include relatively high sensitivity and selectivity for Cl−, insensitivity to changes in bicarbonate concentration and pH, and the possibility of prolonged continuous measurements when using two-photon excitation. It is also important to mention that MQAE is quenched rapidly by Cl− (<1 msec; Verkman et al. 1989) and is thus well suited for monitoring physiological changes in [Cl−]i, often occurring in the millisecond-to-second range. Furthermore, MQAE is quenched by a collisional quenching mechanism, which does not involve binding of Cl− to the indicator dye molecule (Verkman 1990). MQAE, therefore, does not buffer Cl−, and an increase in the intracellular dye concentration improves the signal-to-noise ratio without disturbing the time course of Cl− transients. Furthermore, when using fluorescence lifetime imaging, MQAE becomes a ratiometric dye, allowing quantitative Cl− measurements. The major limitation of MQAE is the loss of the intracellular dye through leakage. The leakage rate seems to be preparation specific, ranging from 3%/h in liposomes (Verkman et al. 1989) to 30%/h in brain slices (Marandi et al. 2002). As can be expected for a lipophilic compound, its leakage rate is temperature dependent. Therefore, the leakage of the dye is very prominent in vivo. This restricts the duration of in vivo Cl− measurements to ∼2 h after the staining procedure. MQAE is a 'non-ratiometric' chloride ion (Cl-)-quenched fluorescent indicator that is used to determine intracellular Cl- concentration ([Cl-]i). MQAE-based two-photon microscopy is reported to be a useful method to measure [Cl-]i, but it is still controversial because a change in cell volume may alter the MQAE concentration, leading to a change in the fluorescence intensity without any change in [Cl-]i. In an attempt to elucidate the effect or lack of effect of cell volume on MQAE concentration, we studied the effects of changes in cell volume, achieved by applying different levels of osmotic stress, on the intensity of MQAE fluorescence in airway ciliary cells. To study solely the effect of changes in cell volume on MQAE fluorescence intensity, i.e., excluding the effect of any change in [Cl-]i, we first conducted the experiments in a Cl--free nitrate (NO3-) solution to substitute NO3- (non-quenching anion for MQAE fluorescence) for Cl- in the intracellular fluid. Hypo- (- 30 mM NaNO3) or hyper-osmotic stress (+ 30 mM NaNO3) effected changes in cell volume, but the stress did not result in any significant change in MQAE fluorescence intensity. The experiments were also carried out in Cl--containing solution. Hypo-osmotic stress (- 30 mM NaCl) increased both MQAE fluorescence intensity and cell volume, while hyper-osmotic stress (+ 30 mM NaCl) decreased both of these properties. These results suggest that the osmotic stress-induced change in MQAE fluorescence intensity was caused by the change in [Cl-]i and not by the MQAE concentration. Moreover, the intracellular distribution of MQAEs was heterogeneous and not affected by the changes in osmotic stress-induced cell volume, suggesting that MQAEs are bound to un-identified subcellular structures. These bound MQAEs appear to have enabled the measurement of [Cl-]i in airway ciliary cells, even under conditions of cell volume change.[4] |
| 分子式 |
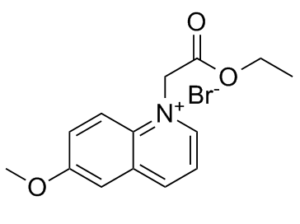
C14H16BRNO3
|
|
|---|---|---|
| 分子量 |
326.19
|
|
| 精确质量 |
325.031
|
|
| 元素分析 |
C, 51.55; H, 4.94; Br, 24.50; N, 4.29; O, 14.71
|
|
| CAS号 |
162558-52-3
|
|
| 相关CAS号 |
|
|
| PubChem CID |
2762651
|
|
| 外观&性状 |
Light yellow to khaki solid powder
|
|
| 熔点 |
177-179ºC(lit.)
|
|
| tPSA |
39.41
|
|
| 氢键供体(HBD)数目 |
0
|
|
| 氢键受体(HBA)数目 |
4
|
|
| 可旋转键数目(RBC) |
5
|
|
| 重原子数目 |
19
|
|
| 分子复杂度/Complexity |
282
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
CCOC(=O)C[N+]1=CC=CC2=C1C=CC(=C2)OC.[Br-]
|
|
| InChi Key |
DSLLHVISNOIYHR-UHFFFAOYSA-M
|
|
| InChi Code |
InChI=1S/C14H16NO3.BrH/c1-3-18-14(16)10-15-8-4-5-11-9-12(17-2)6-7-13(11)15;/h4-9H,3,10H2,1-2H3;1H/q+1;/p-1
|
|
| 化学名 |
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中(例如氮气保护),避免吸湿/受潮和光照。 |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 2.08 mg/mL (6.38 mM) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 悬浮液;超声助溶。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (6.38 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: 100 mg/mL (306.57 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶 (<60°C). 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.0657 mL | 15.3285 mL | 30.6570 mL | |
| 5 mM | 0.6131 mL | 3.0657 mL | 6.1314 mL | |
| 10 mM | 0.3066 mL | 1.5328 mL | 3.0657 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT02254551 | Terminated Has Results | Drug: LDE225 Drug: Bortezomib |
Multiple Myeloma | SCRI Development Innovations, LLC | January 2015 | Phase 2 |
| NCT04066504 | Active, not recruiting | Drug: sonidegib | Basal Cell Carcinoma | Sun Pharmaceutical Industries Limited | March 11, 2019 | |
| NCT02086513 | Terminated | Drug: LDE225 | Graft Versus Host Disease | Massachusetts General Hospital | April 2014 | Phase 1 |
| NCT04007744 | Recruiting | Biological: Pembrolizumab Drug: Sonidegib |
Clinical Stage III Cutaneous Melanoma AJCC v8 Clinical Stage III Gastric Cancer AJCC v8 |
Mayo Clinic | February 13, 2020 | Phase 1 |