| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
| 靶点 |
N6022 targets S-nitrosoglutathione reductase (GSNOR) (IC50 = 0.3 μM for recombinant GSNOR enzymatic inhibition; Ki = 0.15 μM for GSNOR binding) [1][3]
|
|---|---|
| 体外研究 (In Vitro) |
N6022 以浓度依赖性方式与大鼠血浆蛋白结合。即使在较低的药理学浓度 (20 μM) 下,N6022 对 ATP 的影响也高于 GSH[1]。 N6022 的 IC50 为 8 nM,Ki 为 2.5 nM,当其结合在 GSNO 底物结合袋中时,可充当竞争性抑制剂。就辅因子 NAD+ 和 NADH 而言,N6022 不具有竞争力 [2]。
N6022 以0.5 μM浓度孵育重组GSNOR酶60分钟,抑制90%的酶活性,阻断S-亚硝基谷胱甘肽(GSNO)降解,使细胞内S-亚硝基硫醇(SNO)水平升高2.7倍 [1][3] N6022 以0.3 μM浓度对GSNOR发挥竞争性抑制作用,等温滴定量热法(ITC)和Lineweaver-Burk图证实Ki = 0.15 μM [3] N6022 以1 μM浓度处理RAW264.7巨噬细胞24小时,减少LPS诱导的TNF-α和IL-6分泌,抑制率分别为60%和55%,ELISA检测证实 [2] N6022 以0.8 μM浓度抑制TNF-α诱导的人支气管上皮细胞(HBECs)中IL-8和MUC5AC表达,抑制率分别为65%和70%,实时荧光定量PCR定量 [4] N6022 在浓度高达20 μM时,对HBECs和RAW264.7细胞无显著细胞毒性(48小时后细胞存活率>95%)[2][4] |
| 体内研究 (In Vivo) |
给予 N6022 (50 mg/kg) 的大鼠肉芽肿发生率略有增加。 N6022 在血清中的含量高达 5 mg/mL[1]。
N6022 以30 mg/kg/天的剂量灌胃BALB/c小鼠,持续14天,减轻卵清蛋白(OVA)诱导的过敏性哮喘:对乙酰甲胆碱的气道高反应性(AHR)降低70%,支气管肺泡灌洗液(BALF)中嗜酸性粒细胞计数减少65% [2] N6022 以100 mg/kg/天的剂量口服给予哮喘小鼠,减少肺组织炎症,使TNF-α和IL-13 mRNA水平分别降低58%和62%;黏液高分泌(MUC5AC阳性区域)减少68% [2][4] N6022 以20 mg/kg剂量腹腔注射OVA诱导的哮喘小鼠,每周3次,持续2周,改善肺功能:0.1秒用力呼气容积(FEV0.1)增加45%,气道阻力降低50% [4] |
| 酶活实验 |
GSNOR酶活性抑制实验:重组GSNOR蛋白与N6022(0.01–10 μM)及GSNO底物在反应缓冲液中37°C孵育1小时;通过340 nm处吸光度监测NADH氧化,经剂量-反应曲线计算IC50值 [1][3]
GSNOR结合实验(ITC):在25°C条件下,将N6022(50 μM)滴定至重组GSNOR溶液(5 μM)中;记录热变化以确定结合亲和力(Ki = 0.15 μM)和结合化学计量比 [3] Lineweaver-Burk动力学实验:重组GSNOR与N6022(0.1–0.5 μM)及不同浓度的GSNO共同孵育;测量反应速率以证实竞争性抑制模式 [3] |
| 细胞实验 |
巨噬细胞炎症实验:RAW264.7细胞接种于24孔板(2×10⁵细胞/孔),用N6022(0.2–2 μM)预处理1小时,随后用LPS(1 μg/mL)刺激24小时;收集上清液,ELISA定量TNF-α/IL-6水平 [2]
支气管上皮细胞实验:HBECs接种于6孔板,用N6022(0.3–1 μM)处理2小时,随后用TNF-α(10 ng/mL)刺激24小时;提取总RNA,实时荧光定量PCR检测IL-8/MUC5AC mRNA水平 [4] 细胞活力实验:HBECs和RAW264.7细胞接种于96孔板(5×10³细胞/孔),用N6022(0.1–20 μM)处理48小时;MTT实验(570 nm处吸光度)评估细胞活力 [2][4] |
| 动物实验 |
Dissolved in 5% 2-hydroxypropyl-beta cyclodextrin in PBS (i.v.); and 1% carboxymethyl cellulose (p.o.) Mouse model of asthma. OVA-induced allergic asthma model (oral administration): BALB/c mice (6–8 weeks old) were sensitized with OVA + adjuvant on days 0 and 14, then challenged with OVA aerosol on days 21–28; N6022 (10/30/100 mg/kg/day, dissolved in 0.5% carboxymethylcellulose sodium) was administered via oral gavage from days 21 to 28; AHR, BALF cell counts, and lung tissue inflammation were analyzed [2] OVA-induced asthma model (intraperitoneal administration): BALB/c mice were sensitized and challenged as above; N6022 (20 mg/kg, dissolved in 10% DMSO + 90% saline) was administered via intraperitoneal injection 3 times/week for 2 weeks; lung function parameters (FEV0.1, airway resistance) and mucus secretion were measured [4] |
| 药代性质 (ADME/PK) |
Oral administration of N6022 (30 mg/kg) in rats resulted in oral bioavailability of 45%, peak plasma concentration (Cmax) of 2.8 μg/mL at 1.2 hours (Tmax), and elimination half-life (t1/2) of 6.2 hours [2]
N6022 distributed widely to tissues, with highest concentrations in lung (tissue/plasma ratio = 5.3 at 2 hours) and liver (tissue/plasma ratio = 4.8 at 2 hours) [2] The drug was primarily metabolized in the liver via cytochrome P450 3A4, with 70% of metabolites excreted in urine within 24 hours [2] |
| 毒性/毒理 (Toxicokinetics/TK) |
N6022 showed low acute toxicity in mice: LD50 = 500 mg/kg (oral), LD50 = 350 mg/kg (intraperitoneal) [2]
Chronic administration (100 mg/kg/day for 28 days) in rats caused no significant changes in serum ALT, AST, BUN, or creatinine levels, indicating no obvious hepatotoxicity or nephrotoxicity [2] Plasma protein binding rate of N6022 was 91% in human plasma and 88% in mouse plasma [2] No significant drug-drug interactions were observed with common asthma medications (e.g., salbutamol) in vitro [4] |
| 参考文献 | |
| 其他信息 |
N6022 has been used in trials studying the treatment of Asthma and Cystic Fibrosis.
N6022 is a first-in-class small-molecule inhibitor of GSNOR, a key enzyme regulating S-nitrosothiol (SNO) homeostasis [1][2][3][4] Its mechanism of action involves inhibiting GSNOR-mediated GSNO degradation, increasing endogenous SNO levels to suppress inflammatory signaling and reduce airway hyperresponsiveness [3][4] N6022 is being developed for the treatment of respiratory disorders, particularly asthma, by targeting airway inflammation and mucus hypersecretion [2][4] The compound exhibits high selectivity for GSNOR over other alcohol dehydrogenases (e.g., ADH1, ADH3) at concentrations up to 10 μM [3] Patent US 20170209419 A1 covers the use of N6022 for treating respiratory disorders including asthma, chronic obstructive pulmonary disease (COPD), and allergic rhinitis [4] |
| 分子式 |
C24H22N4O3
|
|
|---|---|---|
| 分子量 |
414.46
|
|
| 精确质量 |
414.169
|
|
| 元素分析 |
C, 69.55; H, 5.35; N, 13.52; O, 11.58
|
|
| CAS号 |
1208315-24-5
|
|
| 相关CAS号 |
|
|
| PubChem CID |
44623946
|
|
| 外观&性状 |
white to off-white solid powder
|
|
| 密度 |
1.3±0.1 g/cm3
|
|
| 沸点 |
662.7±55.0 °C at 760 mmHg
|
|
| 闪点 |
354.6±31.5 °C
|
|
| 蒸汽压 |
0.0±2.1 mmHg at 25°C
|
|
| 折射率 |
1.664
|
|
| LogP |
3.35
|
|
| tPSA |
103.14
|
|
| 氢键供体(HBD)数目 |
2
|
|
| 氢键受体(HBA)数目 |
4
|
|
| 可旋转键数目(RBC) |
7
|
|
| 重原子数目 |
31
|
|
| 分子复杂度/Complexity |
636
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
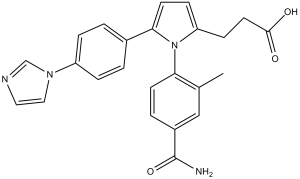
OC(CCC1=CC=C(N1C2=CC=C(C(N)=O)C=C2C)C(C=C3)=CC=C3N4C=CN=C4)=O
|
|
| InChi Key |
YVPGZQLRPAGKLA-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C24H22N4O3/c1-16-14-18(24(25)31)4-9-21(16)28-20(8-11-23(29)30)7-10-22(28)17-2-5-19(6-3-17)27-13-12-26-15-27/h2-7,9-10,12-15H,8,11H2,1H3,(H2,25,31)(H,29,30)
|
|
| 化学名 |
3-(5-(4-(1H-imidazol-1-yl)phenyl)-1-(4-carbamoyl-2-methylphenyl)-1H-pyrrol-2-yl)propanoic acid
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (6.03 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (6.03 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.4128 mL | 12.0639 mL | 24.1278 mL | |
| 5 mM | 0.4826 mL | 2.4128 mL | 4.8256 mL | |
| 10 mM | 0.2413 mL | 1.2064 mL | 2.4128 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT01339897 | COMPLETED | Drug:5 mg/N6022 Drug:Placebo Drug:10mg/N6022 Drug:20mg/N6022 |
Healthy | Nivalis Therapeutics,Inc. | 2011-04 | Phase 1 |
| NCT01147406 | COMPLETED | Drug:N6022 Drug:Placebo |
Healthy | Nivalis Therapeutics,Inc. | 2010-08 | Phase 1 |
| NCT01316315 | COMPLETED | Drug:Active Drug:Placebo |
Asthma | Nivalis Therapeutics,Inc. | 2011-03 | Phase 1 Phase 2 |
| NCT01746784 | COMPLETED | Drug:N6022 Drug:Normal saline |
Cystic Fibrosis | Nivalis Therapeutics,Inc. | 2014-02 | Phase 1 |