| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| Other Sizes |
|
| 靶点 |
ETA (Ki = 0.679 nM); ETB (Ki = 569 nM)
|
|---|---|
| 体外研究 (In Vitro) |
Nebentan 对 [125I]endothelin-1 与内皮素 ETA 和 ETB 受体的特异性结合具有浓度依赖性抑制作用,人和大鼠内皮素 ETA 受体的 Ki 值分别为 0.697 nM 和 1.53 nM。 YM598 对人和大鼠内皮素 ETB 受体的 Ki 值分别为 569 nM 和 155 nM,具有较低的亲和力 [1]。当测量细胞内 Ca2+ 浓度时,Nebentan 浓度依赖性地降低 CHO 细胞和 A10 细胞中 10 nM 内皮素-1 诱导的 [Ca2+]i 增加; CHO 细胞中的 IC50 值分别为 26.2 nM,A10 细胞中的 IC50 值分别为 26.7 nM [1]。
结合/binding分析[1] 使用来自表达人内皮素ETA和ETB受体的CHO细胞以及大鼠细胞和组织的膜,检查了内本坦(YM598)抑制[125I]内皮素-1与内皮素ETA及ETB受体结合的效力。YM598以浓度依赖的方式抑制[125I]内皮素-1与内皮素ETA和ETB受体的特异性结合(图2A和B)。YM598对人和大鼠内皮素ETA受体的Ki值分别为0.697±0.132nM(n=6)和1.53±0.16nM(n=4)。相比之下,YM598对人和大鼠内皮素ETB受体的亲和力较低,Ki值分别为569±90 nM(n=6)和155±11 nM(t=4)。还对波生坦进行了结合实验(图2A和B)。波生坦对人和大鼠内皮素ETA受体的Ki值分别为2.28±0.26 nM(n=6)和7.99±1.68 nM(t=4),对人和鼠内皮素ETB受体的Kis值分别为174±19 nM(=6)和34.9±3.0 nM(=4)。 为了进一步评估Nebentan(YM598)与内皮素-ETA受体之间相互作用的性质,使用表达人内皮素-ETA-受体的CHO细胞膜进行了[125I]内皮素-1饱和结合研究。很明显,增加YM598的浓度会导致线斜率的连续降低(图2C),表明[125I]内皮素-1结合的Kd值发生了一种变化。YM598对Bmax(最大[125I]内皮素-1结合)没有显著影响。 受体特异性[1] 在59次放射性配体结合试验中确定了内本坦(YM598)的特异性。表1显示,除地尔硫卓结合位点外,内本坦(YM598)在10μM时没有显著的抑制活性。然而,在功能测定(KCl诱导的兔主动脉收缩)中,YM598(高达10μM)没有抑制L型Ca2+通道介导的(KCl诱发的)收缩,而地尔硫卓则有(数据未显示)。 体外功能抑制效力:细胞内Ca2+浓度的测定[1] 向表达人内皮素ETA受体的Fura 2负载CHO细胞和大鼠A10细胞中添加内皮素-1导致[Ca2+]i浓度依赖性增加,而沙拉福毒素S6c在高达100 nM的浓度下没有任何作用。在表达人内皮素ETA受体的CHO细胞和大鼠A10细胞中,内皮素-1的EC50值分别为10.5±4.3 nM(n=6)和6.1±3.8 nM(n=6)。在100 nM内皮素-1时达到最大效果(CHO细胞:386±103 nM,大鼠A10细胞:207±32 nM,每个细胞n=6),其数量被认为与其他激动剂诱导的数量几乎相同(Roullet等人,1997)Nebentan(YM598)浓度依赖性地抑制了10 nM内皮素-1在CHO细胞和A10细胞中诱导的[Ca2+]i的增加,CHO细胞(n=6)的IC50值为26.2±3.6 nM,A10细胞(n=6)的IC50值为26.7±8.2 nM(图3)。波生坦还抑制了两种细胞中[Ca2+]i的增加,CHO细胞(n=6)的IC50值为53.5±9.2 nM,A10细胞(n=6)的IC50值为39.4±5.5 nM(图3)。YM598和波生坦对基础[Ca2+]i没有显示出任何激动或拮抗作用。 体外功能抑制效力:主动脉环收缩[1] 内皮素-1以浓度依赖的方式诱导大鼠胸主动脉制备的环收缩(图4)。内皮素-1在该组织中诱导的收缩反应是由内皮素-ETA受体介导的(Panel等人,1992)Nebentan(YM598)(10-1000 nM)拮抗了内皮素-1诱导的血管收缩,而没有降低最大反应(图4),但即使在1000 nM时,对基础张力也没有直接影响。Schild图分析的YM598的pA2值为7.6(95%置信区间:6.5-7.8),斜率为0.84(n=6-8)。 |
| 体内研究 (In Vivo) |
右心室肥厚和肺动脉高压的发展可被nebentan(口服;0.1-1 mg/kg;4周)显着抑制[2]。口服nebentan(1 mg/kg;30周)可显着降低肺充血和双心室肥厚,同时还可改善CHF大鼠的不良生存率[2]。
体内功能抑制效力:对大内皮素-1或沙拉福毒素S6c诱导的清醒正常血压大鼠血压变化的影响[1] 在清醒的正常血压大鼠中,研究了内本坦(YM598)和波生坦对内皮素-1诱导的大升压反应的影响。静脉注射大内皮素-1(0.5 nmol/kg)导致平均血压升高(113.1±3.3至169.5±3.7 mm Hg),在注射后10至15分钟达到峰值,并在清醒大鼠1小时内恢复到基线。口服YM598(0.1、0.3、1mg/kg)30分钟后,平均血压没有变化。口服YM598(0.1、0.3、1 mg/kg)可剂量依赖性地抑制内皮素-1诱导的升压反应,每剂量口服30分钟后观察到最大抑制作用(图6A)。口服1 mg/kg的剂量时,YM598对内皮素-1诱导的大升压反应产生了约80%的抑制作用,约60%的抑制作用持续了至少6.5小时。此外,即使在口服1 mg/kg剂量后24小时,也有约25%的抑制作用(赋形剂:89.7±6.8%,YM598:65.0±9.7%,图6A)。口服波生坦(3、10、30 mg/kg)也能剂量依赖性地抑制大内皮素-1(0.5 nmol/kg)诱导的升压反应,口服给药后30分钟观察到最大的抑制作用(图6B)。然而,与YM598相比,波生坦的口服剂量(30mg/kg)至少需要高出30倍,才能对大内皮素-1的升压反应产生类似程度和持续时间的抑制(图6B)。 体内功能抑制效力:抑制髓内大鼠对大内皮素-1的升压反应[1] 静脉注射大内皮素-1(0.1-3.2 nmol/kg),内皮素-1的前体肽,诱导了剂量依赖性升压反应,并使带髓雄性Wistar大鼠的舒张压最大增加约100 mm Hg(图5)Nebentan(YM598)(0.1、0.3、1 mg/kg)剂量依赖性地抑制了内皮素-1诱导的这种大的升压反应,并在静脉注射和口服给药后产生了平行的右移,DR2值分别为0.53 mg/kg、静脉注射和0.77 mg/kg、口服(图5)。根据这些DR2值计算出的静脉注射/口服比率为0.69。另一方面,波生坦的DR2值为5.1 mg/kg静脉注射和25.2 mg/kg口服,计算出的波生坦静脉注射/口服比为0.20。[1] 在清醒的正常血压大鼠中,还研究了内本坦(YM598)和选择性ETB受体拮抗剂K-8794(Sonoki等人,1997)对选择性ETB激动剂沙拉福毒素S6c诱导的降压和升压反应的影响。静脉注射沙拉福毒素S6c(0.3 nmol/kg)产生了短暂的降压反应(-23.9±1.7 mm Hg),随后是持续的升压反应(30.6±2.1 mm Hg)。口服YM598(1、3、10 mg/kg,口服)在所有剂量下均未显著抑制沙拉福毒素S6c诱导的降压和升压反应(双向方差分析重复测量)(图7A、B)。另一方面,口服K-8794(0.3至30mg/kg)剂量依赖性地抑制了沙拉福毒素S6c诱导的初始降压和升压反应(图7C,D)。口服30mg/kg波生坦也抑制了对沙拉福毒素S6c的降压反应,但没有抑制对沙拉弗毒素S6c(数据未显示)的升压反应。 研究了新型选择性内皮素-A(ETA)受体拮抗剂内本坦(YM598)对双侧心力衰竭的影响。单次皮下注射60mg/kg野百合碱可产生继发于肺动脉高压的右侧心力衰竭,而手术左冠状动脉结扎可产生缺血后充血性左侧心力衰竭(CHF)。在右侧心力衰竭大鼠中,口服Nebentan(YM598)(0.1和1mg/kg,持续4周),但不口服波生坦(30mg/kg),可显著抑制肺动脉高压的进展和右心室肥大的发展。YM598还改善了这些大鼠的低氧血症和形态学肺损伤。此外,在CHF大鼠中,长期口服奈本坦(YM598)(1mg/kg/天,持续约30周)显著改善了其较差的存活率(P<0.05)。在测量心脏血流动力学参数时,YM598将左心室的收缩/舒张能力和右心室的前负荷提高到假手术大鼠的水平。YM598还显著抑制了CHF大鼠的心室肥大和肺充血,并降低了高血浆脑利钠肽水平。这些发现表明,YM598可能在改善右侧心力衰竭的心肺变化以及CHF的心功能障碍和死亡率/发病率方面具有临床益处[2]。 |
| 酶活实验 |
结合分析[1]
内皮素受体结合测定根据Webb等人(1993)的方法进行,如前所述,并进行了修改。简而言之,在250μl的总体积中进行了竞争研究,其中含有25μl[125I]内皮素-1(重组人内皮素ETA和ETB受体为200 pM,大鼠A10和小脑为500 pM)、25μl竞争化合物或100 nM内皮素-1以确定非特异性结合,以及孵育缓冲液(50 mM Tris-HCl,pH 7.4,10 mM MgCl2和0.01%牛血清白蛋白)。通过加入200μl由孵育缓冲液重建的质膜悬浮液引发反应,其中含有0.2μg(重组人内皮素ETA和ETB受体)、24μg(大鼠A10)或2.4μg(鼠小脑)的膜蛋白。孵育期后(重组人内皮素ETA和ETB受体3小时,大鼠A10和小脑2小时,室温),通过加入3毫升冰冷的孵育缓冲液终止反应,然后通过Whatman GF/C过滤器快速过滤。将过滤器冲洗两次,并使用伽马计数器以60%的效率对过滤器上残留的放射性物质进行计数。每次测定进行4-6次,一式两份。对于饱和结合研究,在不存在或存在不同浓度的内本坦(YM598)的情况下,将每种质膜制剂与不同浓度的[125I]内皮素-1(1.5-800 pM)一起孵育。检测条件与竞争结合的描述相同。最大特异性结合计算为总结合减去非特异性结合。通过位移曲线的回归分析确定对[125I]内皮素-1的特异性结合产生50%抑制(IC50)的试验化合物的浓度。抑制解离常数(Ki)由以下公式计算:Ki=IC50/(1+[C]/Kd),其中[C]是管中存在的放射性配体的浓度,Kd是从Scatchard图获得的放射性配体解离常数(Cheng和Prusoff,1973)。 受体特异性[1] 通过在59种不同的配体结合试验中测量Nebentan (YM598)与受体特异性配体竞争的能力,检查了内本坦(YM598)对内皮素受体的特异性。YM598在10μM下进行了测试。 |
| 细胞实验 |
体外功能抑制效力:细胞内Ca2+浓度的测定[1]
根据之前Grynkiewicz等人,1985年,Tahara等人,1998年描述的方法进行细胞内Ca2+浓度([Ca2+]i)的测量,并稍作修改。表达人内皮素ETA受体的CHO细胞和A10细胞被放置在盖玻片(直径13.5 mm)上,血清饥饿12小时。细胞单层在37°C下用Fura 2-AM(4μM/盖玻片)在Hank's平衡盐溶液(HBSS:140 mm NaCl,4 mm KCl,1 mm K2HPO4,1 mm MgCl2,1 mm CaCl2,10 mm葡萄糖,20 mm HEPES,pH 7.4)中装载1小时。然后将它们洗涤,转移到无Fura 2的HBSS中,并在37°C下再孵育30分钟。将盖玻片放入含有2ml HBSS缓冲液的石英比色皿中,并在持续搅拌下保持在37°C。当达到热平衡时,用CAF-110荧光分光光度计记录荧光信号,激发波长为340/380nm,发射波长为500nm。在短暂记录基线信号后,将载体或测试化合物加入试管中。在加入测试化合物两分钟后,将内皮素-1加入试管中,以在有或没有测试化合物的情况下刺激[Ca2+]i的动员。通过用非荧光Ca2+离子载体离子霉素(25μM)测定最大荧光(Rmax),将荧光测量值转换为[Ca2+]i,然后通过添加3 mM EGTA获得最小荧光(Rmin)。根据340和380 nm处的荧光比,使用以下方程计算[Ca2+]i:[Ca2+]i(nm)=Kd×[(R−Rmin)/(Rmax−R)]×b。术语b是Fura 2在0和饱和Ca2+中在380nm处的荧光比。在实验开始和结束时两次得出Rmax、Rmin和b值,并在计算中使用平均值。Kd是Fura 2对Ca2+的解离常数,假设为224 nM。通过将[Ca2+]i的增加表示为内皮素-1(10nM)治疗的百分比来评估测试化合物的活性,在每个实验中使用相同的细胞制剂进行测定。通过回归分析确定试验化合物的IC50值。 体外功能抑制效力:大鼠主动脉环收缩[1] 用分离的大鼠主动脉环评估内皮素-1诱导的血管收缩的拮抗作用,因为这种反应是由该组织中的内皮素-ETA受体介导的(Panek等人,1992)。雄性Wistar大鼠(320-370g)用戊巴比妥钠(60mg/kg i.p.)麻醉,迅速取出胸主动脉,放入95:5 O2/CO2的Krebs-Henseleit溶液(118.4mM NaCl、4.7mM KCl、1.2mM MgCl2、1.2mM KH2PO4、25mM NaHCO3、2.5mM CaCl2、11.1mM葡萄糖)中,以保持pH 7.4。使用小棉球轻轻摩擦内膜表面以去除内皮,并将每个环悬浮在10毫升单独的硅化器官室中,该器官室含有充气(95:5 O2/CO2)和加热(37°C)的Krebs-Henseleit溶液。血管段连接到与自然地理记录仪相连的等距力传感器上,用于监测张力变化。基线张力设定为1.0 g,让组织平衡1小时。组织用苯肾上腺素(1μM)最大限度地收缩,然后用乙酰胆碱(1μM)激发。对乙酰胆碱的负性舒张反应证实了内皮细胞的缺失。在洗掉这些试剂后,用60mM KCl反复刺激环收缩,直到对KCl的收缩反应在开始实验之前变得稳定。在30分钟的预处理期后,在有或没有内本坦(YM598)的情况下,对内皮素-1的累积浓度-反应曲线进行了研究。收缩反应以60mM KCl引发的反应的百分比表示。通过回归分析确定了在存在或不存在内本坦(YM598)的情况下引起50%最大反应(EC50)的内皮素-1的有效浓度。产生浓度-反应曲线向右移动2倍至激动剂(pA2)值所需的拮抗剂摩尔浓度的负对数通过以下方程确定为效力指数:pA2=log(浓度比-1)-log[B],其中浓度比是有和没有拮抗剂的EC50值之比,[B]是拮抗剂的浓度。图对数(浓度比-1)与对数[B](Schild图)的回归分析使我们能够通过评估其斜率来确认拮抗剂的竞争性质。 |
| 动物实验 |
In vivo functional inhibitory potency: inhibition of pressor response to big endothelin-1 in pithed rats [1]
In vivo antagonistic activity in pithed rats was evaluated according to the method of Clozel et al. (1994) as described previously. Briefly, after tracheal intubation, male Wistar rats (280–320 g) were pithed with a steel rod under sodium pentobarbital anesthesia (60 mg/kg, i.p.) and artificially ventilated with room air. The right common carotid artery and the left femoral vein were cannulated for blood pressure measurements and i.v. administration of drugs, respectively. After stabilization of blood pressure, various doses of (1 ml/kg) Nebentan (YM598) or vehicle (distilled water) were injected. Five minutes later, the first dose of big endothelin-1 was injected intravenously in a volume of 0.5 ml/kg. Increasing doses were injected in a cumulative manner (0.1–3.2 nmol/kg, i.v.), with each dose being given after stabilization of the effect of the previous dose on blood pressure. In another series of experiments, the oral activity of Nebentan (YM598) was tested. Various doses of (5 ml/kg) Nebentan (YM598) or vehicle (0.5% methyl cellulose) were orally administered by gastric gavage with a cannula. About 20 min later, the rats were anesthetized with sodium pentobarbital, and 30 min after dosing were pithed and ventilated. About 1 h after oral administration of YM598, waiting for stabilization of blood pressure, the first dose of big endothelin-1 was injected intravenously. In this study, DR2 value was defined as the dose of YM598 required to produce a 2-fold rightward shift of dose–response curves to big endothelin-1 in diastolic blood pressure. In vivo functional inhibitory potency: effect on big endothelin-1- or sarafotoxin S6c-induced changes in blood pressure in conscious normotensive rats [1] Male Wistar rats (270–360 g) were anesthetized with sodium pentobarbital (60 mg/kg i.p.). The right common carotid artery and the left jugular vein were cannulated with a polyethylene tube for determination of blood pressure and heart rate, and for i.v. administration of big endothelin-1. The animals were allowed to recover for 2 days after the operation, during which time they were housed in individual cages with free access to rat chow and water. Rats were then placed in individual cages, and big endothelin-1 (0.5 nmol/kg) was intravenously administered three times at intervals of 1 h. Thirty minutes after the third administration of big endothelin-1, various doses of Nebentan (YM598), bosentan or vehicle (0.5% methyl cellulose) (5 ml/kg) were orally administered by gastric gavage with a cannula. Repeated doses of big endothelin-1 were administered 30 min later, followed every 60 min over a 6-h period and finally again 24 h after administration with Nebentan (YM598), bosentan, or vehicle. The activity of the test compound was evaluated by expressing the pressor response in mean blood pressure as the percentage of that to the third administration of big endothelin-1. In another set of experiments, the effects on sarafotoxin S6c-induced depressor and pressor responses were also tested. Instead of big endothelin-1, sarafotoxin S6c (0.3 nmol/kg) was intravenously administered and various doses of YM598, K-8794, a selective endothelin ETB receptor antagonist (Sonoki et al., 1997), and vehicle were orally administered. The activity of the test compound was evaluated by expressing the depressor and pressor response as the percentage of those to the third administration of sarafotoxin S6c. |
| 参考文献 |
|
| 其他信息 |
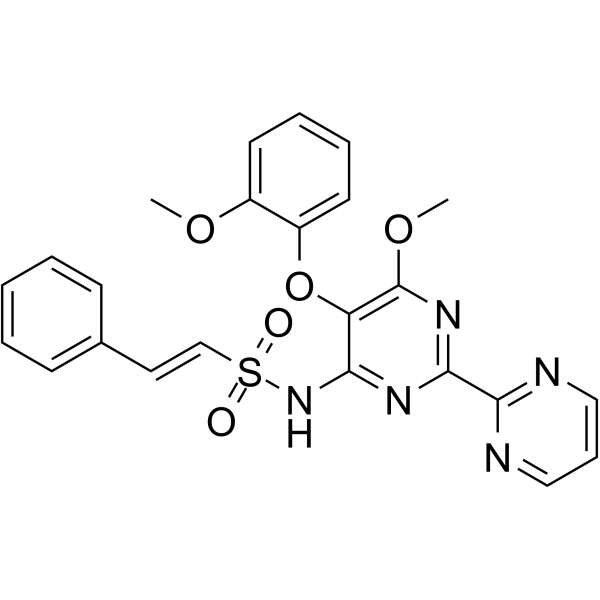
Endothelin Receptor Type A Antagonist YM598 is an orally active synthetic substituted phenylethenesulfonamide. As a selective endothelin A receptor antagonist, YM598 inhibits endothelin-mediated mechanisms involved in tumor cell growth and progression, angiogenesis, and metastasis.
We describe here the pharmacology of (E)-N-[6-methoxy-5-(2-methoxyphenoxy)[2,2'-bipyrimidin]-4-yl]-2-phenylethenesulfonamide monopotassium salt (YM598), a novel selective endothelin ET(A) receptor antagonist synthesized through the modification of the ET(A)/ET(B) non-selective antagonist, bosentan. YM598 inhibited [125I]endothelin-1 binding to cloned human endothelin ET(A) and ET(B) receptor, with K(i) of 0.697 and 569 nM, and inhibited endothelin-1-induced increases in intracellular Ca(2+) concentration in human and rat endothelin ET(A) receptor. YM598 also inhibited endothelin-1-induced vasoconstriction in isolated rat aorta with a pA(2) value of 7.6. In vivo, YM598 inhibited the pressor response to big endothelin-1, a precursor peptide of endothelin-1. DR(2) values of YM598 in pithed rats were 0.53 mg/kg, i.v. and 0.77 mg/kg, p.o., and its antagonism in conscious rats was maintained for more than 6.5 h at 1 mg/kg, p.o. In contrast, YM598 had no effect on the sarafotoxin S6c-induced depressor or pressor responses. YM598 showed not only superior antagonistic activity and higher-selectivity for endothelin ET(A) receptor in vitro, but at least a 30-fold higher potency in vivo than bosentan. In conclusion, YM598 is a potent and orally active selective endothelin ET(A) receptor antagonist. [1] To assess the specificity of YM598 for the endothelin receptor, YM598 was tested at 10 μM in a variety of radioligand competition assays using 59 receptors. YM598 did not affect radioligand binding except at the diltiazem binding site. In a functional assay (KCl-induced contraction in rabbit aorta), however, YM598 did not inhibit KCl-induced contraction. These data indicate that YM598 might not provide functional inhibition of the effects of L-type Ca2+-channel blockers, including diltiazem. As a point of reference, a 10-μM concentration is approximately 10,000-fold higher than the Ki of YM598 for binding at the endothelin ETA receptor. These data show the specificity of YM598 for the endothelin receptor. The ability of YM598 to antagonize endothelin-1-induced functional responses in vitro was investigated by measuring the inhibitory effects of endothelin-1 on the increases in [Ca2+]i in both CHO cells expressing human endothelin ETA receptor and rat A10 cells. YM598 concentration-dependently antagonized the increase in [Ca2+]i stimulated by 10 nM endothelin-1. Almost identical IC50 values in both cells indicate that the antagonistic activity of YM598 in humans is equal to that in rats. We also evaluated the effect of YM598 on the contractile response of isolated rat aorta for endothelin ETA receptor-mediated contraction, because endothelin-1 is a potent constrictor of smooth muscle and the endothelin ETA receptor is the predominant mediator of endothelin-1 activity in rat aorta (Panek et al., 1992). YM598 produced a parallel and rightward shift of the endothelin-1 concentration–response curve without affecting the maximal force to yield a pA2 value of 7.6. These results indicate that YM598 is a functional endothelin ETA receptor antagonist. To evaluate the antagonistic effect of YM598 in vivo, we examined the effects of both intravenous and oral administration of YM598 in rats. The pressor response induced by exogenous big endothelin-1 is considered to be mediated by the endothelin ETA receptor in rats (Haleen et al., 1993). In pithed rats, intravenous and oral administration of YM598 produced a parallel rightward shift of big endothelin-1-induced dose–response curves, with DR2 values of 0.53 mg/kg, i.v. and 0.77 mg/kg, p.o. The calculated i.v./p.o. ratio from these DR2 values was 0.69. In contrast, DR2 values of i.v. and p.o. administration and calculated i.v./p.o. ratio of bosentan were 5.1 mg/kg, 25.2 mg/kg, and 0.20, which are almost equal to previously reported data (Clozel et al., 1994). The relative potencies of YM598 to bosentan in inhibiting exogenous big endothelin-1 were approximately 10-fold on i.v. and 30-fold on p.o. administration. These results indicate that YM598 has potent antagonistic activity in vivo as well as in vitro and may be easily absorbed by oral administration with a higher oral bioavailability than bosentan. We also evaluated the inhibitory effect of YM598 on the pressor response to exogenous big endothelin-1 in conscious normotensive rats at the same doses used in pithed rats. The magnitude of maximum inhibition was about 55%, 70%, and 80% at the doses of 0.1, 0.3, and 1 mg/kg of YM598, 30 min after oral administration. At the same doses, the duration of action was 2.5 h, over 6.5 h, and over 24 h, while a 30-fold higher oral dose of bosentan was needed to bring about a response equal to that of YM598. These results suggest that YM598 has a long-lasting effect and that its oral antagonistic activity to endothelin response via the endothelin ETA receptor is 30-fold more potent than that of bosentan in vivo. Finally, to ascertain whether YM598 retains endothelin ETA receptor selectivity in vivo, we investigated the effects of YM598 on sarafotoxin S6c-induced responses in conscious normotensive rats, and compared the results with those of K-8794, a selective endothelin ETB receptor antagonist. Oral administration of YM598 at up to 10 mg/kg affected neither the depressor nor pressor responses to sarafotoxin S6c. However, K-8794 potently suppressed depressor and pressor responses to sarafotoxin S6c at 1 to 10 mg/kg oral administration. This result is consistent with those previously reported Sonoki et al., 1997, Sawaki et al., 2000, which showed K-8794 to be ineffective on the pressor response to big endothelin-1 at 30 mg/kg in rats. These results indicate that YM598 is a selective endothelin ETA receptor antagonist in vivo, at least up to 10 mg/kg. In conclusion, we have developed a new selective endothelin ETA receptor antagonist, YM598, through the modification of bosentan. YM598 is a potent, orally active endothelin ETA receptor antagonist with a long duration of action. This compound blocks endothelin ETA receptors but not endothelin ETB receptors both in vitro and in vivo, and its endothelin ETA receptor antagonistic activities were more potent than those of bosentan. Although it has not been clarified whether blockade of endothelin ETA receptors only or of both endothelin ETA and ETB receptors is more beneficial in the long-term treatment of diseases associated with endothelins, the potency and selectivity of YM598 provide a new tool in analyzing the pathophysiological role of the endothelin ETA receptor in various disorders in which endothelins are implicated. [1] |
| 分子式 |
C24H21N5O5S
|
|---|---|
| 分子量 |
491.5190
|
| 精确质量 |
491.126
|
| CAS号 |
403604-85-3
|
| 相关CAS号 |
Nebentan potassium;342005-82-7
|
| PubChem CID |
9957262
|
| 外观&性状 |
Light yellow to yellow solid powder
|
| LogP |
5.309
|
| tPSA |
133.8
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
10
|
| 可旋转键数目(RBC) |
9
|
| 重原子数目 |
35
|
| 分子复杂度/Complexity |
767
|
| 定义原子立体中心数目 |
0
|
| SMILES |
S(/C(/[H])=C(\[H])/C1C([H])=C([H])C([H])=C([H])C=1[H])(N([H])C1=C(C(=NC(C2N=C([H])C([H])=C([H])N=2)=N1)OC([H])([H])[H])OC1=C([H])C([H])=C([H])C([H])=C1OC([H])([H])[H])(=O)=O
|
| InChi Key |
LONWRQOYFPYMQD-DTQAZKPQSA-N
|
| InChi Code |
InChI=1S/C24H21N5O5S/c1-32-18-11-6-7-12-19(18)34-20-21(29-35(30,31)16-13-17-9-4-3-5-10-17)27-23(28-24(20)33-2)22-25-14-8-15-26-22/h3-16H,1-2H3,(H,27,28,29)/b16-13+
|
| 化学名 |
(E)-N-[6-methoxy-5-(2-methoxyphenoxy)-2-pyrimidin-2-ylpyrimidin-4-yl]-2-phenylethenesulfonamide
|
| 别名 |
Nebentan; 403604-85-3; Nebentan [INN]; UNII-IJ670B0H4A; IJ670B0H4A; HE-11; DTXSID50193350; (E)-N-(6-Methoxy-5-(2-methoxyphenoxy)-2,2'-bipyrimidin-4-yl)-2-phenylethenesulfonamide;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~125 mg/mL (~254.31 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (4.23 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (4.23 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL 澄清 DMSO 储备液加入到 900 μL 玉米油中并混合均匀。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.0345 mL | 10.1725 mL | 20.3451 mL | |
| 5 mM | 0.4069 mL | 2.0345 mL | 4.0690 mL | |
| 10 mM | 0.2035 mL | 1.0173 mL | 2.0345 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。