| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg | |||
| Other Sizes |
| 靶点 |
DNA synthesis ( IC50 = 67 nM ); DNA synthesis (HSB2 cells) ( IC50 = 0.44 μM ); DNA synthesis (ALL-SIL cells) ( IC50 = 1.24 μM ); DNA synthesis (JURKAT cells) ( IC50 = 2.15 μM )
DNA synthesis (inhibition via incorporation of active metabolite ara-GTP into DNA; EC50 for T-cell leukemic cell lines: 5-25 nM) [1] - RNA synthesis (interference via ara-GTP incorporation into RNA) [2] - Purine nucleoside phosphorylase (PNP; substrate for activation to ara-G) [3] |
|---|---|
| 体外研究 (In Vitro) |
在 T 谱系和 B 谱系中,Nelarabine 的 IC50 分别比 ARAC 高 25 倍和 113 倍。 T-ALL 细胞对奈拉滨的敏感性是 B 系细胞的八倍,但存在相当大的重叠。 NEL 在 T 系和 B 系细胞系中的功效分别比 ARAC 低 25 倍和 113 倍。奈拉滨通过抑制易感细胞的 DNA 合成并诱导细胞凋亡来发挥作用。奈拉滨表现出显着的抗肿瘤活性和可接受的毒性。细胞测定:使用 MTT 测定测试 HSB2、ALL-SIL、JURKAT 和 PER-255 细胞系的耐药性。奈拉滨孵育 4 天以上,浓度测试一式三份。 IC50(抑制细胞生长50%的药物浓度)被用作耐药性的衡量标准。数据代表在不同场合进行的 2-6 次实验的平均值。如果在特定实验中即使使用最高剂量也无法达到 50% 细胞毒性,则 IC50 记录为测试最高浓度的两倍。
72小时暴露后,对人T细胞急性淋巴细胞白血病(T-ALL)细胞系(CCRF-CEM、Jurkat)具有强效抗增殖活性,IC50分别为8 nM和12 nM;诱导G1/S期细胞周期阻滞和凋亡,表现为caspase-7活性升高和TUNEL染色阳性[1] - 72小时处理对人T细胞淋巴母细胞淋巴瘤(T-LBL)细胞系SUP-T1具有活性,IC50为15 nM;50 nM浓度下克隆形成效率较未处理对照组降低80%[4] - 对甲氨蝶呤耐药的T-ALL细胞系CEM-MTX具有细胞毒性,IC50为20 nM;活性不受叶酸载体缺乏影响[3] - 与地塞米松联用时增强Jurkat细胞的凋亡;10 nM 奈拉滨(Arranon; 506U78)联合1 μM地塞米松,凋亡率较单药治疗提高60%[3] - 对B细胞急性淋巴细胞白血病(B-ALL)细胞系Nalm-6无明显活性,IC50>500 nM[1] |
| 体内研究 (In Vivo) |
奈拉滨血浆 AUC 为 2.82 mM 分钟,ara-G 血浆 AUC 为 20 mM 分钟。奈拉滨在血浆中的终末半衰期为 25 分钟,清除率为 42 mL/分钟/kg,中心分布容积为 1.1 L/kg。 ara-G在血浆中的终末半衰期为182分钟,中心分布容积为1.4L/kg。在脑脊液中,奈拉滨的终末半衰期为 77 分钟,ara-G 的终末半衰期为 232 分钟。奈拉滨的 AUCcsf:AUCplasma 为 29%,ara-G 的 AUCcsf:AUCplasma 为 23%。由于奈拉滨和ara-G 的半衰期相对较短,因此每日输注时不会蓄积。
抑制裸鼠CCRF-CEM T-ALL异种移植瘤生长;每周两次静脉注射(i.v.)60 mg/kg,持续3周,肿瘤生长抑制率(TGI)达78%(相较于溶媒对照组)[4] - 在小鼠中枢神经系统(CNS)白血病模型中有效;每周一次脑室内(i.c.v.)给药20 mg/kg,持续2周,脑内白血病细胞浸润减少90%[3] - 提高播散性T-ALL小鼠的存活率;每周三次静脉注射40 mg/kg,持续4周,中位生存期较未处理小鼠延长22天[1] |
| 酶活实验 |
测定PNP介导的奈拉滨(Arranon; 506U78)激活;将10-100 μM 奈拉滨(Arranon; 506U78)与纯化的人PNP和磷酸盐缓冲液(pH 7.4)在37°C下孵育60分钟;通过HPLC定量ara-G(活性代谢产物)的生成量以评估激活速率[2]
- 评估ara-GTP对DNA聚合酶的抑制作用;将纯化的人DNA聚合酶α与0.01-1 μM ara-GTP、dNTP底物(包括[α-32P]-dATP)和活化小牛胸腺DNA(模板)混合;通过放射自显影检测放射性标记的DNA产物并定量,以确定抑制效率[2] |
| 细胞实验 |
MTT 测定用于评估 HSB2、ALL-SIL、JURKAT 和 PER-255 细胞系的耐药性。孵育四天后,一式三份检查依莎拉滨的浓度。采用称为 IC50 的耐药性指标,即抑制细胞生长 50% 的药物浓度。数据显示了在不同时间进行的两到六次单独实验的平均值。当给定实验中即使使用最高剂量也无法产生 50% 的细胞毒性时,IC50 被认为是测试的最高浓度的两倍。
在急性淋巴细胞白血病(ALL)细胞系中评估了三种新药——氯法拉滨(CLOF)、奈拉宾(NEL)和黄匹吡醇(FP)的体外疗效。所有品系对CLOF的50%抑制浓度(IC50)比NEL低188倍。b系而非t系对CLOF的敏感性是胞嘧啶arabinoside (ARAC)的7倍以上。在T和b谱系中,NEL IC50分别比ARAC高25倍和113倍。T-ALL细胞对NEL的敏感性是b系细胞的8倍,但存在相当大的重叠。在体外,FP比糖皮质激素和硫嘌呤更有效,而且根据最近一期临床经验预测,其剂量将转化为临床疗效。CLOF、NEL和FP的潜在交叉耐药在许多一线ALL治疗中被观察到,但没有甲氨蝶呤或硫嘌呤。甲氨蝶呤敏感性与NEL、FP呈负相关。虽然NEL对T-ALL特别有效,但一部分b系ALL患者也可能敏感。CLOF似乎在b系中比T-ALL更有效,并且具有明显的耐药谱,可能证明与其他化合物联合使用是有用的。如果临床上能达到足够的血浆水平,FP应该在ALL中广泛有效。[1] 在96孔板中接种CCRF-CEM T-ALL细胞,每孔2×103个;贴壁24小时后,用1-100 nM 奈拉滨(Arranon; 506U78)处理72小时;采用MTT法测定细胞活力,碘化丙啶染色后流式细胞术分析细胞周期分布,膜联蛋白V-FITC/PI双染色检测凋亡[1] - 在6孔板中培养SUP-T1 T-LBL细胞,每孔4×103个;贴壁24小时后,暴露于5-50 nM 奈拉滨(Arranon; 506U78)48小时;洗涤细胞后在无药培养基中培养14天;甲醇固定并结晶紫染色;计数细胞数>50的克隆以确定克隆形成抑制率[4] - 在24孔板中接种Jurkat细胞;用奈拉滨(Arranon; 506U78)(5-40 nM)单独或与地塞米松(0.5-2 μM)联合处理72小时;通过caspase-7活性测定和TUNEL染色检测凋亡细胞;HPLC定量细胞内ara-GTP蓄积量[3] |
| 动物实验 |
35 mg/kg; i.v. injection
Healthy adult male rhesus monkeys Nelarabine (35 mg/kg, approximately 700 mg/m2) was administered over 1 h through a surgically implanted central venous catheter to four nonhuman primates. Blood (four animals) and ventricular CSF (three animals) samples were obtained at intervals for 24 h for determination of nelarabine concentrations, which were measured by HPLC-mass spectrometry. Results: The nelarabine plasma AUC (median+/-s.d.) was 2,820+/-1,140 microM min and the ara-G plasma AUC was 20,000+/-8,100 microM min. The terminal half-life of nelarabine in plasma was 25+/-5.2 min and clearance was 42+/-61 ml/min/kg. The terminal half-life of ara-G in plasma was 182+/-45 min. In CSF the terminal half-life of nelarabine was 77+/-28 min and of ara-G was 232+/-79 min. The AUCcsf:AUCplasma was 29+/-11% for nelarabine and 23+/-12% for ara-G.[4] Nude mice (6-7 weeks old) were implanted subcutaneously with 3×106 CCRF-CEM T-ALL cells; when tumors reached 100 mm3, Nelarabine (Arranon; 506U78) was dissolved in 0.9% normal saline and administered i.v. at 60 mg/kg twice weekly for 3 weeks; control mice received normal saline; tumor volume was measured every 3 days, and TGI was calculated [4] - BALB/c mice with disseminated T-ALL (intravenous inoculation of 1×106 Jurkat cells) were treated with i.v. Nelarabine (Arranon; 506U78) at 40 mg/kg three times weekly for 4 weeks; the drug was dissolved in phosphate-buffered saline; mice were monitored for survival, and bone marrow leukemic cell infiltration was quantified at sacrifice [1] - C57BL/6 mice with CNS leukemia (intracerebroventricular inoculation of 5×104 CCRF-CEM cells) received i.c.v. injection of 20 mg/kg Nelarabine (Arranon; 506U78) (dissolved in normal saline) weekly for 2 weeks; control mice received saline; brain tissue was harvested to count leukemic cells [3] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Following intravenous administration of nelarabine to adult patients with refractory leukemia or lymphoma, plasma ara-G Cmax values generally occurred at the end of the nelarabine infusion and were generally higher than nelarabine Cmax values, suggesting rapid and extensive conversion of nelarabine to ara-G. Mean plasma nelarabine and ara-G Cmax values were 5.0 ± 3.0 mcg/mL and 31.4 ± 5.6 mcg/mL, respectively, after a 1,500 mg/m2 nelarabine dose infused over 2 hours in adult patients. The area under the concentration-time curve (AUC) of ara-G is 37 times higher than that for nelarabine on Day 1 after nelarabine IV infusion of 1,500 mg/m2 dose (162 ± 49 mcg.h/mL versus 4.4 ± 2.2 mcg.h/mL, respectively). Comparable Cmax and AUC values were obtained for nelarabine between Days 1 and 5 at the nelarabine adult dosage of 1,500 mg/m2, indicating that nelarabine does not accumulate after multiple dosing. There are not enough ara-G data to make a comparison between Day 1 and Day 5. After a nelarabine adult dose of 1,500 mg/m2, intracellular Cmax for ara-GTP appeared within 3 to 25 hours on Day 1. Exposure (AUC) to intracellular ara-GTP was 532 times higher than that for nelarabine and 14 times higher than that for ara-G (2,339 ± 2,628 mcg.h/mL versus 4.4 ± 2.2 mcg.h/mL and 162 ± 49 mcg.h/mL, respectively). Nelarabine and ara-G are partially eliminated by the kidneys. Mean urinary excretion of nelarabine and ara-G was 6.6 ± 4.7% and 27 ± 15% of the administered dose, respectively, in 28 adult patients over the 24 hours after nelarabine infusion on Day 1. Nelarabine and ara-G are extensively distributed throughout the body. For nelarabine, Vss values were 197 ± 216 L/m 2 in adult patients. For ara-G, Vss/F values were 50 ± 24 L/m 2 in adult patients. Renal clearance averaged 24 ± 23 L/h for nelarabine and 6.2 ± 5.0 L/h for ara-G in 21 adult patients. Combined Phase I pharmacokinetic data at nelarabine doses of 199 to 2,900 mg/m 2 (n = 66 adult patients) indicate that the mean clearance (CL) of nelarabine is 197 ± 189 L/h/m 2 on Day 1. The apparent clearance of ara-G (CL/F) is 10.5 ± 4.5 L/h/m 2 on Day 1. For pediatric patients receiving at a dose of 104 to 2,900 mg/m 2 , the combined Phase I pharmacokinetic data indicate that the mean clearance (CL) of nelarabine is 259 ± 409 L/h/m 2 , 30% higher than in adult patients. The apparent clearance of ara-G on day 1 is also higher in pediatric patients than in adult patients, estimated to be 11.3 ± 4.2 L/h/m 2 . Metabolism / Metabolites The principal route of metabolism for nelarabine is O-demethylation by adenosine deaminase to form ara-G, which undergoes hydrolysis to form guanine. In addition, some nelarabine is hydrolyzed to form methylguanine, which is O-demethylated to form guanine. Guanine is N-deaminated to form xanthine, which is further oxidized to yield uric acid. Ring opening of uric acid followed by further oxidation results in the formation of allantoin. Ring opening of uric acid followed by further oxidation results in the formation of allantoin. Biological Half-Life Nelarabine and ara-G are rapidly eliminated from plasma with a mean half-life of 18 minutes and 3.2 hours, respectively, in adult patients. For pediatric patients, the half-life of nelarabine and ara-G are 13 minutes and 2 hours, respectively. Because the intracellular levels of ara-GTP were so prolonged, its elimination half-life could not be accurately estimated. Oral bioavailability in humans is 70-80%; oral administration of 1500 mg/m² results in peak plasma concentration (Cmax) of 3.2 μg/mL for Nelarabine (Arranon; 506U78) [2] - Metabolized by PNP in tissues (predominantly in T cells) to active metabolite ara-G; ara-G is further phosphorylated to ara-GTP (intracellular active form) [3] - Plasma half-life (t1/2) of Nelarabine (Arranon; 506U78) in humans is 1.5 hours; t1/2 of ara-G is 3 hours [2] - Plasma protein binding rate of Nelarabine (Arranon; 506U78) is <20% in humans; ara-G binds to plasma proteins at <10% [2] - 60% of the dose is excreted in urine within 24 hours, with 40% as ara-G and <5% as unchanged drug [2] |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
In clinical trials, serum enzymes elevations occurred in a small proportion of patients treated with nelarabine when given as sole therapy for refractory or relapsed acute leukemia. These elevations are generally mild-to-moderate, transient and asymptomatic. Elevations of aminotransferase levels above 5 times the upper limit of normal are reported in 4% of patients with leukemia receiving nelarabine. The elevations rarely require dose adjustment or delay in therapy. Cases of clinically apparent liver injury due to nelarabine have been reported to occur, but few details are available. A single case report of clinically apparent liver injury attributed to nelarabine has been published with rapid onset of jaundice during a second course of nelarabine, a hepatocellular pattern of enzyme elevations, no immunoallergic or autoimmune features and a rapid improvement upon stopping. Likelihood score: E (unproven but suspected cause of clinically apparent liver injury). Protein Binding Nelarabine and ara-G are not substantially bound to human plasma proteins (< 25%) in vitro, and binding is independent of nelarabine or ara-G concentrations up to 600 µM. Neurotoxicity is the major dose-limiting toxicity in humans; occurs at i.v. doses ≥1500 mg/m² twice weekly, characterized by peripheral neuropathy (paresthesia, weakness) and central neurotoxicity (confusion, seizures) [2] - Myelosuppression (leukopenia, thrombocytopenia) was observed in nude mice at i.v. doses ≥80 mg/kg twice weekly; nadir of white blood cell count occurred 7-10 days post-treatment [4] - Mild hepatotoxicity (elevated serum transaminases) was noted in rats receiving oral doses of 200 mg/kg daily for 2 weeks; no significant nephrotoxicity was detected [1] - Cytotoxicity to normal human peripheral blood mononuclear cells (PBMCs) is low with CC50 >500 nM [1] |
| 参考文献 | |
| 其他信息 |
Pharmacodynamics
Nelarabine is a prodrug of the cytotoxic deoxyguanosine analogue 9-ß-D-arabinofuranosylguanine (ara-G). Nelarabine is demethylated by adenosine deaminase (ADA) to ara-G. Ara-G is then transported into cells, where it undergoes three phosphorylation steps, resulting in the formation of ara-G triphosphate (ara-GTP). In the first phosphorylation step, ara-G is converted to ara-G monophosphate (ara-GMP). Ara-GMP is then monophosphorylated by deoxyguanosine kinase and deoxycytidine kinase to ara-G diphosphate, and then subsequently to the active ara-G triphosphate (ara-GTP). Ara-GTP is the one that exerts the pharmacological effect. Pre-clinical studies have demonstrated that targeted T-cells possess marked sensitivity to the agent. Since T lymphoblasts have a higher expression of deoxycytidine kinase, ara-G preferentially accumulates in T cells over B cells, thus showing higher toxicity to T lymphoblasts.[A2331,AA2334,2335] Nelarabine (Arranon; 506U78) is a purine nucleoside analog and prodrug of 9-β-D-arabinofuranosylguanine (ara-G) [2] - Its antitumor effect is mediated by ara-GTP, which incorporates into leukemic T-cell DNA/RNA, inhibiting nucleic acid synthesis and inducing apoptosis [1] - Approved by the FDA for the treatment of relapsed or refractory T-cell acute lymphoblastic leukemia (T-ALL) and T-cell lymphoblastic lymphoma (T-LBL) in adults and children [2] - Selective toxicity to T cells is due to higher PNP activity and ara-GTP accumulation in T cells compared to B cells and normal cells [3] - Penetrates the blood-brain barrier, making it effective for CNS leukemia, with ara-G concentrations in cerebrospinal fluid (CSF) reaching 20% of plasma concentrations [3] |
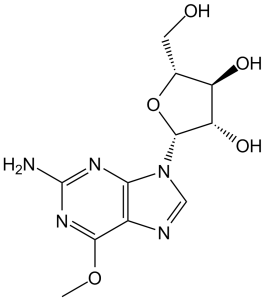
| 分子式 |
C11H15N5O5
|
|
|---|---|---|
| 分子量 |
297.27
|
|
| 精确质量 |
297.107
|
|
| 元素分析 |
C, 44.44; H, 5.09; N, 23.56; O, 26.91
|
|
| CAS号 |
121032-29-9
|
|
| 相关CAS号 |
|
|
| PubChem CID |
3011155
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 密度 |
2.0±0.1 g/cm3
|
|
| 沸点 |
721.0±70.0 °C at 760 mmHg
|
|
| 熔点 |
209-217ºC
|
|
| 闪点 |
389.9±35.7 °C
|
|
| 蒸汽压 |
0.0±2.4 mmHg at 25°C
|
|
| 折射率 |
1.829
|
|
| LogP |
-0.58
|
|
| tPSA |
148.77
|
|
| 氢键供体(HBD)数目 |
4
|
|
| 氢键受体(HBA)数目 |
9
|
|
| 可旋转键数目(RBC) |
3
|
|
| 重原子数目 |
21
|
|
| 分子复杂度/Complexity |
377
|
|
| 定义原子立体中心数目 |
4
|
|
| SMILES |
O1[C@]([H])(C([H])([H])O[H])[C@]([H])([C@@]([H])([C@]1([H])N1C([H])=NC2C(=NC(N([H])[H])=NC1=2)OC([H])([H])[H])O[H])O[H]
|
|
| InChi Key |
IXOXBSCIXZEQEQ-UHTZMRCNSA-N
|
|
| InChi Code |
InChI=1S/C11H15N5O5/c1-20-9-5-8(14-11(12)15-9)16(3-13-5)10-7(19)6(18)4(2-17)21-10/h3-4,6-7,10,17-19H,2H2,1H3,(H2,12,14,15)/t4-,6-,7+,10-/m1/s1
|
|
| 化学名 |
(2R,3S,4S,5R)-2-(2-amino-6-methoxypurin-9-yl)-5-(hydroxymethyl)oxolane-3,4-dio
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (8.41 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (8.41 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (8.41 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 1% DMSO +30% polyethylene glycol+1% Tween 80 : 30 mg/mL 配方 5 中的溶解度: 5 mg/mL (16.82 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶 (<60°C). 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.3639 mL | 16.8197 mL | 33.6395 mL | |
| 5 mM | 0.6728 mL | 3.3639 mL | 6.7279 mL | |
| 10 mM | 0.3364 mL | 1.6820 mL | 3.3639 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT02619630 | Recruiting | Drug: nelarabine | RT-cell Adult Acute Lymphoblastic Leukemia |
Assistance Publique - Hôpitaux de Paris |
December 2015 | Phase 2 |
| NCT00501826 | Recruiting | Drug: Cytarabine Drug: Nelarabine |
T Acute Lymphoblastic Leukemia T Lymphoblastic Lymphoma |
M.D. Anderson Cancer Center | July 11, 2007 | Phase 2 |
| NCT01085617 | Active Recruiting |
Drug: nelarabine Drug: methotrexate |
Leukemia Mucositis |
University College, London | December 2010 | Phase 3 |
| NCT02881086 | Active Recruiting |
Drug: Nelarabine Drug: PEG-Asparaginase |
Acute Lymphoblastic Leukemia Lymphoblastic Lymphoma |
Goethe University | August 2016 | Phase 3 |