| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg | |||
| 50mg |
|
||
| 100mg | |||
| 250mg | |||
| 500mg | |||
| Other Sizes |
| 靶点 |
5-HT1A Receptor ( Ki = 3.4 nM )
F-15599 is a selective agonist at 5-HT1A receptors (Ki = 0.8 nM for rat 5-HT1A receptors). It shows >100-fold selectivity over 5-HT1B, 5-HT1D, 5-HT2A, 5-HT2C, D1, D2, and α1-adrenoceptors. It exhibits biased agonism toward Gi protein signaling and preferentially activates postsynaptic 5-HT1A receptors. [3] |
|---|---|
| 体外研究 (In Vitro) |
F-15599 (NLX-101)在放射性配体结合实验中显示对5-HT1A受体的选择性比其他血清素受体亚型高100倍[3]。
显示偏向性激动特征,优先激活Gαi/o而非β-arrestin信号通路[2]。 |
| 体内研究 (In Vivo) |
F15599(0.06 或 0.12 mg/kg)显着降低 l-DOPA 诱导的运动障碍 (LID),而不影响大鼠的运动表现。 F15599高剂量30 μg/μL治疗的大鼠表现出较少的LID和轻微的5-HT综合征[1]。 F15599(0.0625、0.125、0.25、0.5 和 1.0 mg/kg,i,p)导致攻击潜伏期显着且剂量依赖性(MED = 0.125 mg/kg)延迟,并有效降低(ID50 = 0.095 mg/kg)针对入侵大鼠的攻击行为量。从 0.25 mg/kg 剂量开始,F15599 会诱发所谓的血清素综合征的明显症状,其特征是扁平身体姿势、摇头、下唇缩回和后肢外展,导致行为不活动得分增加和社交脱离[2]。 F15599 使内侧前额叶皮层 (mPFC) 锥体神经元的放电率从 0.2 µg/kg iv 增加,并在剂量 >10 倍时降低中缝背侧 5-羟色胺能神经元的放电率(最小有效剂量 8.2 µg/kg iv)。 F15599 增加 mPFC 中的多巴胺输出(依赖于突触后 5-HT1A 受体激活的效应),腹腔注射 ED50 为 30 µg/kg,同时减少海马 5-HT 释放(完全依赖于 5-HT1A 自身受体激活的效应) ED50 为 240 µg/kg ip[3]。
F-15599(0.16 mg/kg 皮下注射)使大鼠前额叶皮层多巴胺水平增加 200%,同时降低背侧中缝核的血清素水平,证实其优先作用于突触后受体。该效应可被 5-HT1A 拮抗剂 WAY100635 阻断。 [3] 在攻击行为大鼠模型(居住者-入侵者测试)中,F-15599(0.04–0.63 mg/kg 皮下注射)在 0.16 mg/kg 剂量下显著减少攻击持续时间和频率。该抗攻击效应可被 WAY100635 逆转,表明由 5-HT1A 受体介导。 [2] F-15599(0.0025–0.04 mg/kg 静脉注射)可诱导大鼠下唇收缩(LLR)和扁平体姿(FBP),但其对突触后反应(ED50 = 0.005 mg/kg)的效力是突触前效应(ED50 = 0.016 mg/kg)的 3 倍。 [3] |
| 动物实验 |
In summary, this model involves housing male rats alone in sizable observation cages measuring 80 × 55 × 50 cm, along with an oviduct-ligated female, to prevent social isolation and promote sexual behavior, which in turn encourages territorial behavior. A baseline level of aggressive and offensive behavior is assessed after a week, three days in a row, during a maximum 10-minute confrontation with a male conspecific who is not known to the subject (WTG rats). About 60 minutes before the start of this social provocation test, the female partner of the experimental rat is taken out of the home observation cage. As conspecific intruder animals, naive male WTG rats (average weight 372 ± 9.5 g, 3.5–4 months old) are socially housed in groups of three in transparent Makrolon type IV cages. The intruder is eliminated during the first three tests as soon as the resident launches their first complete attack, and the attack latency time (ALT) is recorded. The fourth baseline test, which is conducted on day 4, records and analyzes the entire spectrum of behaviors. Based on the ALT and the degree of offensive behavior, the experimental groups are matched. The experimental groups contain only animals that have attacked (ALT <600 s). Eleven of the fourteen4 participants in the study are not attackers. The following day, day 5, the experimental vehicle (sterile Ultra Pure water, n = 19 and n = 20) or F15599 (0.0625, 0.125, 0.25, 0.5 and 1.0 mg/kg, IP, experiment 1, n = 45) or F13714 (0.003, 0.006, 0.012, 0.025, 0.062, 0.125, 0.250, IP, experiment 2, n = 50) are given 30 minutes prior to the 10-minute confrontation with a drug-free unfamiliar intruder conspecific, and their behavior is recorded once more. In addition, animals are tested in experiment 3 thirty minutes after treatment with vehicle/vehicle (UP), vehicle/F15599 (0.1 mg/kg) or F13714, or vehicle/WAY-100635 (0.3 mg/kg) or vehicle or WAY100635 (0.3 mg/kg)/F15599 (0.1 mg/kg) or F13714. There are 6–8 subjects in each treatment group, for a total of 41 creatures.
For aggression studies, F-15599 was dissolved in saline and administered subcutaneously (s.c.) at doses of 0.04, 0.16, and 0.63 mg/kg 30 minutes before behavioral testing. Control groups received saline. [2] For neurochemical and behavioral assays, F-15599 was injected intravenously (i.v.) at 0.0025–0.04 mg/kg or subcutaneously at 0.16 mg/kg. WAY100635 (0.16 mg/kg s.c.) was administered 20 minutes prior to F-15599 for antagonism tests. [3] |
| 参考文献 |
|
| 其他信息 |
F-15599 is a high-efficacy "biased agonist" that preferentially targets cortical postsynaptic 5-HT1A receptors over somatodendritic autoreceptors, enhancing frontal cortex dopamine release. This contrasts with classical 5-HT1A agonists like 8-OH-DPAT. [3]
Its anti-aggressive effects are mediated via postsynaptic 5-HT1A receptors in corticolimbic regions, suggesting therapeutic potential for impulse control disorders. [2] First-in-class biased agonist with preferential postsynaptic activity, developed for neuropsychiatric disorders [3]. |
| 分子式 |
C19H21CLF2N4O
|
|---|---|
| 分子量 |
394.85
|
| 精确质量 |
394.137
|
| 元素分析 |
C, 57.80; H, 5.36; Cl, 8.98; F, 9.62; N, 14.19; O, 4.05
|
| CAS号 |
635323-95-4
|
| 相关CAS号 |
635323-95-4; 955112-72-8; 635323-96-5 (fumarate)
|
| PubChem CID |
11741361
|
| 外观&性状 |
White to off-white solid powder
|
| LogP |
3.64
|
| tPSA |
58.12
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
6
|
| 可旋转键数目(RBC) |
5
|
| 重原子数目 |
27
|
| 分子复杂度/Complexity |
496
|
| 定义原子立体中心数目 |
0
|
| SMILES |
ClC1=C(C([H])=C([H])C(=C1[H])C(N1C([H])([H])C([H])([H])C(C([H])([H])N([H])C([H])([H])C2N=C([H])C(C([H])([H])[H])=C([H])N=2)(C([H])([H])C1([H])[H])F)=O)F
|
| InChi Key |
WAAXKNFGOFTGLP-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C19H21ClF2N4O/c1-13-9-24-17(25-10-13)11-23-12-19(22)4-6-26(7-5-19)18(27)14-2-3-16(21)15(20)8-14/h2-3,8-10,23H,4-7,11-12H2,1H3
|
| 化学名 |
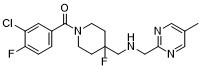
(3-chloro-4-fluorophenyl)-[4-fluoro-4-[[(5-methylpyrimidin-2-yl)methylamino]methyl]piperidin-1-yl]methanone
|
| 别名 |
NLX-101; NLX 101; 635323-95-4; F-15,599; (3-Chloro-4-fluorophenyl)(4-fluoro-4-((((5-methylpyrimidin-2-yl)methyl)amino)methyl)piperidin-1-yl)methanone; UNII-83481Y1YCX; 4-Piperidinemethanamine, 1-(3-chloro-4-fluorobenzoyl)-4-fluoro-N-((5-methyl-2-pyrimidinyl)methyl)-; 83481Y1YCX; F-15,599; NLX101; F-15,599; F 15,599; F15,599; F15599
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 本产品在运输和储存过程中需避光。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: ~250 mg/mL (~633.2 mM)
Ethanol: ~79 mg/mL |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.17 mg/mL (5.50 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 21.7 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.17 mg/mL (5.50 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 21.7 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.17 mg/mL (5.50 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.5326 mL | 12.6630 mL | 25.3261 mL | |
| 5 mM | 0.5065 mL | 2.5326 mL | 5.0652 mL | |
| 10 mM | 0.2533 mL | 1.2663 mL | 2.5326 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
|
|
|
|