| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
δ Opioid Receptor/DOR; mAChR1
|
|---|---|
| 体外研究 (In Vitro) |
脑渗透代谢物 N-去甲基氯氮平优先与 M1 毒蕈碱受体结合,IC50 为 55 nM,并且是比氯氮平更有效的部分激动剂(EC50、115 nM 和 50% 乙酰胆碱反应)[1]。N-desmethylclozapine对 M1 mAChR 具有轻微的激动作用,并对大脑皮层和海马中的 5-HT1A 受体具有激动作用。该化合物还充当大脑皮层和纹状体中 δ-阿片受体的激动剂[2]。 N-去甲基氯氮平 (3 μM) 大大降低兴奋性神经元的外向电流,但不降低抑制性神经元的外向电流。在兴奋性神经元中,单独使用 N-去甲基氯氮平比单独使用氯氮平或氯氮平与 N-去甲基氯氮平的组合更有效。 0.1 μM 哌仑西平和 1 μM 阿托品可显着抑制 N-去甲基氯氮平对兴奋性神经元的作用。 N-去甲基氯氮平(而非氯氮平)通过兴奋性细胞中的 M1 受体抑制 K+ 通道[3]。在未刺激条件下以及 TSST-1 刺激下,N-去甲基氯氮平会导致 TxB2 水平降低。氯氮平、N-去甲基氯氮平和 CPZ 可能通过调节 TxA2 或 TxB2 的产生来作用于神经递质系统 [5]。 N-去甲基氯氮平、盐酸氟西汀和沙美特罗希那菲特在感染 DENV-2 的 Huh-7 细胞中的 IC50 值分别为 1 μM、0.38 μM 和 0.67 μM。与 DMSO 处理相比,用所有三种抑制剂处理的细胞中 NS3 的水平均降低,表明抑制剂在病毒蛋白翻译之前的阶段发挥作用。 N-去甲基氯氮平处理的细胞显示负链 RNA 水平降低了 75% 以上[6]。
非典型抗精神病药氯氮平广泛用于治疗难治性精神分裂症患者。氯氮平及其主要活性代谢产物N-desmethylclozapine/N-去甲基氯氮平(NDMC)具有复杂的药理学特性,并与各种神经递质受体相互作用。有几项生化研究报告称,NDMC在人重组M1毒蕈碱受体上表现出部分激动剂特征。然而,显示NDMC激活完整神经元中天然M1受体的能力的直接电生理证据很差。我们之前使用大鼠海马神经元证明,毒蕈碱激动剂oxotremorine M(oxo-M)激活毒蕈碱受体会导致-40mV时外向K(+)电流的减少。在本研究中,我们使用这种毒蕈碱电流反应评估了氯氮平和NDMC在完整海马兴奋性和抑制性神经元中天然毒蕈碱受体上的激动剂和拮抗剂活性。M1拮抗剂哌仑西平对氧代-M诱导的电流反应的抑制仅在兴奋性神经元中明显,而M3拮抗剂达非那新对这两种类型的神经元都有效。NDMC的毒蕈碱激动剂活性高于氯氮平,兴奋性神经元的活性高于抑制性神经元,对哌仑西平敏感,与氯氮平合用时部分被掩盖。氯氮平的毒蕈碱拮抗剂活性以及NDMC在兴奋性和抑制性神经元之间没有差异,但氯氮平比NDMC更有效。这些结果表明,NDMC具有激活海马兴奋性神经元中表达的天然M1受体的能力,但由于存在过量具有毒蕈碱拮抗剂活性的氯氮平,其激动剂活性在氯氮平治疗的患者中可能受到限制。[3] 血栓素A2(TxA2)及其受体的激活已被证明可以调节血管收缩和血小板聚集,以及多巴胺能和5-羟色胺能信号传导。多巴胺能和5-羟色胺能系统在精神分裂症的病理生理学中起着至关重要的作用,这些系统是抗精神病药物(AP)的主要靶点。由于第一种抗精神病药物氯丙嗪(CPZ)已被证明可以降低TxA2,我们假设AP氯氮平及其代谢产物N-desmethylclozapine/N-去甲基氯氮平(NDMC)也可能影响TxA2的产生。我们使用毒性休克综合征毒素-1(TSST-1)和抗表面抗原CD3与蛋白CD40的单克隆抗体(OKT3/CD40)作为兴奋剂,在全血测定中测量了10名健康女性受试者的未刺激和刺激血液样本中非常不稳定的分子TxA2的代谢产物血栓素B2(TxB2)的水平。血液中补充了四种不同浓度的APs-CPZ、氯氮平或NDMC。此外,在不同刺激条件下,在不添加AP的情况下测量血液中的血栓素水平。在TSST-1和OKT3/CD40刺激下,氯氮平在所有应用浓度下均显著降低了TxB2的平均浓度(p<0.05)。NDMC导致未刺激条件下和TSST-1刺激下TxB2水平降低。在未刺激和TSST-1刺激的条件下,CPZ在低浓度下降低了TxB2的产生。氯氮平、NDMC和CPZ可能通过调节TxA2或TxB2的产生作用于神经递质系统。此外,AP的已知副作用,如直立性低血压,可能是TxA2或TxB2浓度变化的结果。[5] 每年约有1万人死于严重的登革热,世界人口的三分之二生活在登革热流行的地区。登革热病毒疫苗开发取得了显著进展;然而,目前还没有获得许可的登革热抗病毒药物,而且似乎也没有一种正在进行临床试验。我们采取了通过筛选药理学活性化合物库来重新定位已批准的抗登革热病毒活性药物的方法。基于感染细胞数量和病毒滴度的减少,我们确定N-desmethylclozapine/N-去甲基氯氮平、盐酸氟西汀和沙美特罗新酸盐为登革热病毒抑制剂。在抑制剂处理的细胞中,登革热病毒RNA水平降低,这种作用是登革热病毒特有的,因为其他黄病毒,如日本脑炎病毒和西尼罗河病毒,或其他RNA病毒,如呼吸道合胞病毒和轮状病毒,不受这些抑制剂的影响。所有三种抑制剂都以高纳摩尔范围内的50%抑制浓度(IC50)特异性抑制登革热病毒复制。对负链RNA中间体的估计和添加时间实验表明,抑制发生在进入后阶段,最有可能发生在病毒RNA复制的起始阶段。最后,我们表明抑制最有可能是由于内溶酶体途径的调节和自噬的诱导[6]。 |
| 体内研究 (In Vivo) |
大鼠和人体内的 N-去甲基氯氮平/N-desmethylclozapine在 M2 和 M4 mAChR 上分别进行突触前调节 GABA 和谷氨酸释放。特别是,N-desmethylclozapine/N-去甲基氯氮平可能是大鼠中的 M2 mAChR 拮抗剂,但在人新皮质中对该受体没有活性。然而,N-去甲基氯氮平在人类中对 M4 mAChR 有激动作用,但在大鼠新皮质中没有这种作用[4]。
氯氮平是典型的抗精神病药物,其活性部分由氯氮平及其主要代谢产物N-desmethylclozapine/N-去甲基氯氮平(NDMC)组成。先前的研究表明,NDMC在改善氯氮平治疗的精神分裂症患者的认知方面可能比专利化合物本身更重要。虽然氯氮平和NDMC的药理学在大多数方面相似,但NDMC已被证明是M1毒蕈碱受体部分激动剂,而氯氮平在体外和体内都是M1拮抗剂。我们假设,NDMC可能通过直接刺激M1受体增加内侧前额叶皮层(mPFC)中多巴胺(DA)和乙酰胆碱(ACh)的释放来改善认知,而NDMC和氯氮平本身也会通过其他机制这样做,氯氮平会抑制NDMC的M1激动剂作用。在本研究中,我们在清醒、自由活动的大鼠中使用微透析,发现10和20毫克/千克剂量的NDMC显著增加了mPFC和HIP中DA和ACh的释放,但在伏隔核(NAC)中没有。M1偏好拮抗剂telenzepine(3mg/kg)完全阻断了NDMC(10mg/kg)诱导的皮质DA和ACh释放的增加。氯氮平(1.25 mg/kg)本身对皮质中DA或ACh的释放没有影响,它阻断了NDMC(10 mg/kg)诱导的ACh在mPFC中的释放,但不阻断DA的释放。5-HT1A受体拮抗剂WAY100635(0.2 mg/kg)阻断了NDMC(20 mg/kg)诱导的皮质DA,但不阻断ACh的释放。这些发现表明:(1)NDMC是M1激动剂,而氯氮平在体内是M1拮抗剂;(2)NDMC的M1激动作用有助于皮质ACh和DA的释放;(3)NDMC由于其M1激动作用,可能比氯氮平本身更有效地治疗精神分裂症中观察到的认知障碍;(4)M1受体激动可能是开发可以改善精神分裂症认知缺陷的药物的有价值的靶点,也可能是其他神经精神疾病的靶点。[1] 黄氨酸对所有脑区的M1毒蕈碱乙酰胆碱受体(mAChR)以及大脑皮层和海马的5-HT1A受体具有激动活性。另一方面,N-去甲基氯氮平对M1 mAChR表现出轻微的激动作用,并对大脑皮层和海马中的5-HT1A受体表现出激动作用。该化合物还表现为大脑皮层和纹状体δ-阿片受体的激动剂。此外,N-去甲基氯氮平对[(35)S]GTPγS与Gαi/o结合的刺激作用部分是通过mAChR(最有可能是M4 mAChR亚型)介导的,至少在纹状体中是这样。 结论:对天然脑组织中表达的mAChRs(特别是M1亚型,也可能是M4亚型)、5-HT1A受体和δ-阿片受体的激动作用,其中一些是这两种化合物共有的,另一些则是两者的特异性,可能形成了这两种化合物在治疗精神分裂症患者方面的独特有益效果。这些特征为我们开发新的抗精神病药物提供了线索,这些药物超越了多巴胺D2受体拮抗的框架,不仅对阳性症状有效,而且对阴性症状和/或认知/情感障碍也有效。[2] 胆碱能传递在学习、记忆和认知中起着关键作用,胆碱能传递的紊乱与阿尔茨海默病、癫痫和精神分裂症等神经系统疾病有关。包括N-desmethylclozapine/N-去甲基氯氮平(NDMC)在内的药物对这些疾病的药物缓解在动物模型中很有希望,但在患者身上往往失败。因此,我们比较了NDMC对大鼠和人类新皮层切片中谷氨酸能和GABA能传递的影响。我们使用卡巴胆碱(CCh;一种已建立的代谢型毒蕈碱乙酰胆碱(ACh)受体(mAChRs)激动剂)作为参考。使用标准电生理学方法,包括细胞内和场电位记录。在大鼠新皮层中,NDMC阻止了CCh诱导的GABAA和GABAB受体介导的反应的减少,但没有阻止CCh导致的成对脉冲抑制的增加。NDMC既不降低兴奋性突触后电位(EPSP)的振幅,也不拮抗CCh诱导的EPSP抑制。然而,在人类新皮层中,NDMC未能阻止CCh诱导的GABAB反应的减少,并直接降低了EPSP的振幅。这些数据表明,NDMC在M2和M4 mAChRs分别对GABA和谷氨酸释放的突触前调节有明显的影响。特别是,NDMC可能是大鼠的M2 mAChR拮抗剂,但在人类新皮层中对该受体没有活性。然而,NDMC在人类中对M4 mAChR具有激动作用,但在大鼠新皮层中没有这种作用。本研究证实,mAChRs的药理学在物种之间可能存在差异,并强调了对人体组织进行研究的必要性[4]。 |
| 细胞实验 |
抑制剂治疗。[6]
用Huh-7和A549细胞进行登革热病毒感染和抑制剂治疗实验,并通过BHK-21细胞的空斑试验测定培养上清液中的病毒滴度。从8μM浓度开始,使用2倍稀释的抑制剂进行IC50(50%抑制浓度)实验。将总共30000个细胞铺在48孔板中,以3的感染复数(MOI)感染DENV-2,并与含有抑制剂的2%Dulbecco改良Eagle培养基(DMEM)一起孵育。感染后24小时收集上清液(p.i.),通过BHK-21细胞的空斑试验估算病毒滴度。对于蛋白质印迹,在下文所述的抑制剂处理条件下,如前所述制备细胞裂解物(10),并用识别DENV非结构蛋白3(NS3)(Raj Bhatnagar的一种礼物)、微管蛋白单克隆抗体、钙网蛋白和78 kDa葡萄糖调节蛋白(GRP78)抗体探测膜。对于LC3检测,使用对LC3-II型具有高特异性的抗体(LC3b[D11];细胞信号技术)。通过化学发光检测信号。(流式细胞术实验的描述见补充材料。) |
| 动物实验 |
Drugs [1]
NDMC/N-desmethylclozapine and clozapine was dissolved in a small amount of 0.1 M tartaric acid and the pH was adjusted to 6–7 with 0.1 N NaOH. WAY100635 (Wyeth Laboratories, Philadelphia, PA) and telenzepine (Research Chemical Inc.) were dissolved in deionized water. Vehicle or drugs in a volume of 1.0 ml/kg were administered subcutaneously to randomly assigned rats. At 3–5 days after cannulation, a dialysis probe was implanted into the mPFC and NAC under slight anesthesia with isoflurane. Rats were then housed individually overnight in a dialysis cage. After the overnight perfusion at 0.4 μl/min of the probe, the flow was increased to 1.5 μl/min. After 1 h, the dialysate samples were collected every 30 min. The perfusion medium was Dulbecco's phosphate-buffered saline solution, including Ca2+ (138 mM NaCl, 8.1 mM Na2HPO4, 2.7 mM KCl, 1.5 mM KH2PO4, 0.5 mM MgCl, 1.2 mM CaCl2, pH 7.4). No AChesterase inhibitor in the dialysate is required with this procedure (Ichikawa et al, 2002b). After stable baseline values in the dialysates were obtained, each rat received two injections, vehicle/NDMC (N-desmethylclozapine), WAY100635/NDMC, telenzepine/NDMC, or clozapine/NDMC. The locations of the dialysis probes were verified at the end of each experiment by brain dissection. Background: 3(3-Hexyloxy-1,2,5-thiadiazol-4-yl)-1,2,5,6-tetrahydro-1-methylpyridine (xanomeline) and N-desmethylclozapine are of special interest as promising antipsychotics with better efficacy, especially for negative symptoms and/or cognitive/affective impairment. Methods: The guanosine-5'-O-(3-[(35)S]thio)triphosphate ([(35)S]GTPγS) binding experiments were performed using (1) conventional filtration technique, (2) antibody-capture scintillation proximity assay, and (3) immunoprecipitation method, in brain membranes prepared from rat cerebral cortex, hippocampus, and striatum.[2] |
| 药代性质 (ADME/PK) |
Metabolism / Metabolites
N-Desmethylclozapine has known human metabolites that include Desmethylclozapine N-glucuronide. N-Desmethylclozapine is a known human metabolite of clozapine. |
| 参考文献 |
|
| 其他信息 |
N-desmethylclozapine is a dibenzodoazepine substituted with chloro and piperazino groups which is a major metabolite of clozapine; a potent and selective 5-HT2C serotonin receptor antagonist. It has a role as a metabolite, a delta-opioid receptor agonist and a serotonergic antagonist. It is a dibenzodiazepine, a member of piperazines and an organochlorine compound.
ACP-104, or N-desmethylclozapine, is the major metabolite of clozapine, and is being developed by ACADIA as a novel, stand-alone therapy for schizophrenia. It combines an atypical antipsychotic efficacy profile with the added potential benefit of enhanced cognition, thereby addressing one of the major challenges in treating schizophrenia today. Drug Indication Investigated for use/treatment in schizophrenia and schizoaffective disorders. Mechanism of Action ACP-104 combines M1 muscarinic agonism, 5-HT2A inverse agonism, and D2 and D3 dopamine partial agonism in a single compound. ACP-104 uniquely stimulates brain cells known as M1 muscarinic receptors that play an important role in cognition. ACP-104 is a partial-agonist that causes weak activation of dopamine D2 and D3 receptors. These partial agonist properties of ACP-104 may lead to less motoric side effects than seen with most other antipsychotic drugs. Pharmacodynamics ACP-104 is a small molecule drug candidate we are developing as a novel therapy for schizophrenia. It is known that large amounts of ACP-104, or N-desmethylclozapine, are formed in the body after administration of clozapine. That is, clozapine is metabolized to ACP-104. We discovered that ACP-104 has a unique ability to stimulate m1 muscarinic receptors, a key muscarinic receptor. The m1 muscarinic receptors are widely known to play an important role in cognition. Since clozapine itself blocks the m1 muscarinic receptor, patients need to extensively metabolize clozapine into ACP-104 to stimulate this receptor and thereby overcome the blocking action of clozapine. Administration of ACP-104 will avoid the variability of this metabolic process and the competing action of clozapine. Like clozapine, ACP-104 is a dopamine antagonist and a 5-HT2A inverse agonist. We believe that ACP-104 represents a new approach to schizophrenia therapy that combines an atypical antipsychotic efficacy profile with the added advantage of beneficial cognitive effects. The main findings of the present study are that (1) NDMC, the major active metabolite of clozapine, significantly increased DA and ACh release in the mPFC and HIP, but not the NAC; (2) the M1-preferring antagonist telenzepine completely blocked DA and ACh release in the mPFC produced by NDMC; (3) NDMC (10 mg/kg)-induced ACh release was completely blocked by clozapine (1.25 mg/kg), consistent with previous reports that NDMC is a potent M1 agonist, while clozapine has M1 antagonist properties in vivo; (4) clozapine pretreatment did not block NDMC-induced cortical DA release, indicating M1 agonism did not contribute to this effect of NDMC; and (5) the increases in DA, but not ACh, release in the mPFC produced by NDMC was partially blocked by the 5-HT1A antagonist WAY100635, indicating that cortical DA release is partially dependent upon 5-HT1A-receptor stimulation. The effect of clozapine on DA or ACh release is most likely the result of the combined effect of clozapine and NDMC, the agonist/antagonist mixing. Thus, high NDMC levels, and particularly high NDMC/clozapine ratios, would increase M1 muscarinic receptor stimulation, as predicted by mass action and by agonist/antagonist mixing studies (Brauner-Osborne et al, 1996). Brain clozapine concentrations in the rat during chronic treatment have been reported to exceed those of NDMC during chronic treatment by three-fold (Weigmann et al, 1999). There is no information on what the relative levels are in man. High concentrations of NDMC are found in plasma samples in some patients treated with clozapine (Hasegawa et al, 1993). High NDMC levels, and a high NDMC/clozapine ratio even more so, would increase M1 muscarinic receptor stimulation. The present data on the blockade of NDMC-induced ACh release by clozapine are consistent with clinical data from our laboratory, which suggest that the NDMC/clozapine ratio is a better predictor of clinical response to clozapine than clozapine levels alone (Frazier et al, 2003; Mauri et al, 2003; Weiner et al, 2004). In conclusion, NDMC preferentially increased DA and ACh release in the mPFC and HIP but not the NAC, similar to the effect of clozapine and other atypical APDs. The blockade of NDMC-induced ACh release by telezenpine and clozapine indicates that the stimulation of M1 receptors contributes to the ability of NDMC to increase cortical DA and ACh release, confirming that NDMC has significant M1 agonistic actions, whereas the parent compound, clozapine, is an antagonist.[1] In the present study, we assessed agonist and antagonist activities of clozapine and its major active metabolite, NDMC, at native muscarinic receptors in rat intact hippocampal excitatory and inhibitory neurons, by monitoring muscarinic current responses. Our data clearly demonstrated that clozapine acts primarily as an antagonist, whereas NDMC acts as a mixed agonist–antagonist. The agonist activity of NDMC, which was explained by its action on the M1, rather than M3, subtype, was largely masked when the same concentration of clozapine was co-applied. These results suggest that the clozapine/NDMC concentration ratio in clozapine-treated patients might determine the degree of M1 receptor activation by NDMC.[3] In conclusion, the present data cast some doubts on extrapolating from rat neurons to human neurons, when going beyond the standard compounds, because the pharmacology of NDMC differs markedly in the two species (Thomas et al. 2010). Thus, the present study emphasizes the need of studies in human tissue, despite the inherent limitations of these tissues.[4] |
| 分子式 |
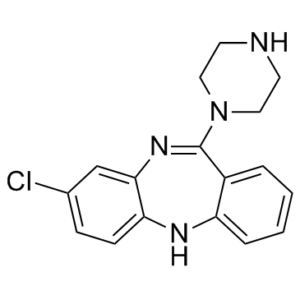
C17H17CLN4
|
|---|---|
| 分子量 |
312.797
|
| 精确质量 |
312.114
|
| 元素分析 |
C, 65.28; H, 5.48; Cl, 11.33; N, 17.91
|
| CAS号 |
6104-71-8
|
| 相关CAS号 |
N-Desmethylclozapine-d8; 1189888-77-4; N-Desmethylclozapine-d8 hydrochloride; 2705402-91-9
|
| PubChem CID |
135409468
|
| 外观&性状 |
Light yellow to green yellow solid powder
|
| 密度 |
1.38g/cm3
|
| 沸点 |
490.1ºC at 760 mmHg
|
| 熔点 |
120-125°C
|
| 闪点 |
250.2ºC
|
| 折射率 |
1.709
|
| LogP |
3.22
|
| tPSA |
39.66
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
3
|
| 可旋转键数目(RBC) |
1
|
| 重原子数目 |
22
|
| 分子复杂度/Complexity |
421
|
| 定义原子立体中心数目 |
0
|
| SMILES |
ClC1=CC=C2C(N=C(N3CCNCC3)C4=CC=CC=C4N2)=C1
|
| InChi Key |
JNNOSTQEZICQQP-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C17H17ClN4/c18-12-5-6-15-16(11-12)21-17(22-9-7-19-8-10-22)13-3-1-2-4-14(13)20-15/h1-6,11,19-20H,7-10H2
|
| 化学名 |
3-chloro-6-piperazin-1-yl-11H-benzo[b][1,4]benzodiazepine
|
| 别名 |
AZD-5991; AZD-5991 S-enantiomer; N-Desmethylclozapine; Norclozapine; 6104-71-8; Desmethylclozapine; N-desmethyl clozapine; N-desmethyl-clozapine; N-DEMETHYLCLOZAPINE; 8-Chloro-11-(1-piperazinyl)-5H-dibenzo(b,e)(1,4)diazepine; AZD 5991 S-enantiomer.
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: ≥ 50 mg/mL (~159.9 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (7.99 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (7.99 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.1969 mL | 15.9847 mL | 31.9693 mL | |
| 5 mM | 0.6394 mL | 3.1969 mL | 6.3939 mL | |
| 10 mM | 0.3197 mL | 1.5985 mL | 3.1969 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT00628420 | Completed | Drug: ACP-104 Drug: Placebo |
Schizophrenia | University of Texas Southwestern Medical Center |
January 2005 | Phase 1 |
| NCT00490516 | Completed | Drug: ACP-104 Drug: Placebo |
Schizophrenia | ACADIA Pharmaceuticals Inc. | June 2007 | Phase 2 |
|
|
|