| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 2mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
HCV (IC50 = 0.82 to 19.3 pM); HCV (IC50 = 366 pM)
|
|---|---|
| 体外研究 (In Vitro) |
体外活性:Ombitasvir 以前称为 ABT-267)是一种基于 N-苯基吡咯烷的丙型肝炎病毒(HCV)NS5A 抑制剂,具有出色的效力、代谢稳定性和药代动力学。奥比他韦被发现是一种泛基因型 HCV 抑制剂,针对 GT1a、-1b、-2a、-2b、-3a、-4a 和 -5a 的 EC50 范围为 1.7-19.3 pM,针对 GT6a 的 EC50 范围为 366 pM。在首次接受治疗的 HCV GT1 感染受试者中,在 3 天的单一治疗期间,它可将 HCV RNA 降低高达 3.10 log10 IU/mL。 Ombitasvir 已被美国食品药品监督管理局 (FDA) 批准与其他抗病毒药物(帕立瑞韦、利托那韦和达沙布韦)联合使用,商品名为 Viekira Pak。激酶测定:之前描述了在HCV复制子细胞培养测定中用于测量单个氨基酸变体对抑制剂活性的影响的方法。简言之,使用 Change-IT 多突变定点诱变试剂盒(Affymetrix,圣克拉拉)将 NS5A 中的抗性相关变体各自引入基因型 1a-H77 或 1b-Con1 或嵌合基因型 2 至 6 复制子之一中。 ,加利福尼亚州)。通过序列分析确认变体的存在后,将质粒线性化,并使用 TranscriptAid T7 高产转录试剂盒(Fermentas,Glen Burnie,MD)从质粒转录 HCV 亚基因组 RNA。在瞬时测定中,含有变体的复制子 RNA 通过电穿孔转染至 Huh-7 细胞系中。 EC50 的计算方法如前几节所述。细胞测定:构建了带有荧光素酶报告基因但不含 Neo 基因的基于基因型 1b-Con1 HCV 复制子的穿梭载体盒,用于评估源自感染 HCV 基因型 1 至 6 的个体的 NS5A 基因的表型。克隆了 NotI 限制位点NS4B 3'端NS5A上游90个核苷酸处插入1b-Con1亚基因组复制子载体,并在NS5A氨基酸413密码子后克隆ClaI位点。来自基因 1 型感染患者的 NS5A 区域插入到 NotI 和 ClaI 限制性位点之间。具有 NotI 和 BlpI 限制性位点(上一节中描述)的 1b-Con1 穿梭载体用于评估 ombitasvir 抑制包含非基因型 1 HCV 氨基酸 1 至 214 的 NS5A 区域的能力。临床样本中的 NS5A 基因被扩增并连接到穿梭载体中。在瞬时测定中,来自每个临床样本的含有 NS5A 基因的 HCV 亚基因组复制子 RNA 通过电穿孔转染至 Huh-7 衍生细胞系中。将细胞在ombitasvir存在下孵育4天。测量细胞中的荧光素酶活性,并如上所述计算EC50。
Ombitasvir (ABT267)是一种丙型肝炎病毒(HCV)NS5A抑制剂,具有皮摩尔效力、泛基因型活性,对HCV基因型1至5的50%有效浓度(EC50s)为0.82至19.3 pM,对基因型6a的有效浓度为366 pM。尽管NS5A中存在天然序列多样性,但Ombitasvir对69个基因型1至6个嵌合复制子仍保持了这些水平的效力,这些复制子包含来自HCV感染患者的NS5A基因。体外耐药性选择鉴定了基因型1至6中HCV NS5A基因氨基酸位置28、30、31、58和93处对ombitasvir产生耐药性的变体。 HCV复制子中的活性。[1] DeGoey等人先前提出了Ombitasvir (ABT267)的活性。Ombitasvir对基因型1a-H77和1b-Con1亚基因组复制子的EC50s分别为14.1和5.0 pM。在40%的人血浆中,由于血浆蛋白结合,化合物被隔离,ombitasvir的抗病毒活性减弱了11至13倍。ombitasvir的CC50大于32μM,导致体外治疗指数超过200万倍。Ombitasvir表现出广泛的基因型活性,对基因型2a、2b、3a、4a和5a复制子的EC50s为0.82至19.3 pM,对基因基因型6a复制子的EC 50s为366 pM(表1)。 在基因型1a、1b、2a、2b、3a、4a、5a和6a复制子中选择对Ombitasvir (ABT267)耐药的变体。[1] 表2和表3列出了ombitasvir在基因型1至6中选择的氨基酸变体。DeGoey等人之前简要介绍了基因型1中ombitasvir的抗性特征。在基因型1a中,ombitasvr在EC50的10倍、100倍或100倍上选择的主要变异是M28T、M28V、Q30R、Y93C和Y93H。在基因型1a-H77背景下,M28V具有58倍的抗性,而M28T、Q30R、Y93C和Y93H变体各自具有超过800倍的抗性。ombitasvir在基因型1b中选择的主要变异是Y93H,对ombitasvr具有77倍的抗性。与基因型1a的观察结果相反,基因型1b中氨基酸位置28、30和31的单个替换赋予了<10倍的抗性。在ombitasvir EC50的100倍或1000倍时,许多克隆含有双氨基酸置换,主要是Y93H,以及NS5A N端区域的额外置换,双变体对ombitasir的抗性超过400倍。 在基因型2至5的EC50以上50倍和基因型6a细胞系的Ombitasvir(ABT267)EC50以上10倍进行抗性选择,因为在高于这些值的浓度下,复制子细胞在G418存在下无法存活,表明细胞中的复制子已被清除。 在基因型2a中,所选的主要变异是T24A和F28S,在基因型2b中,所选择的主要变异为L31V和Y93H,而在24个克隆中只有一个观察到L28F变异。在欧洲HCV数据库中,基因型2a和2b中的氨基酸位置31是多态的,蛋氨酸和亮氨酸都很普遍。因此,一些抗性变体是在M31和L31的背景下构建的。在基因型2a中,发现T24A(加M31)和T24A(加L31)对Ombitasvir (ABT267)的抗性水平相似。基因型2b L28F(加L31)和L31V变体分别具有47倍和511倍的抗性;然而,发现M31背景中的变体L28F对ombitasvir具有248倍的抗性。在基因型3a中检测到的主要抗性相关变异是Y93H,它对ombitasvir的抗性是6728倍。在基因型4a中,唯一选择的变异是L28V,它对ombitasvir具有23倍的抗性。在基因型5a中,观察到变异L28I、L31F和L31V,其中L28I对ombitasvir的抗性为79倍,而L31F和L3 1V变异的抗性均超过240倍。在基因型6a中,选择了L31V和T58的几个变体,这些变体对ombitasvir具有18至101倍的抗性。 总之,在NS5A中,基因型1至6之间观察到的关键抗性相关氨基酸位置为28、30、31、58和93;然而,这些氨基酸位置的变体对Ombitasvir (ABT267)的抗性因基因型而异。图2显示了野生型基因型1至6复制子中NS5A的氨基酸1至100的比对,突出了每个基因型中与抗性相关的标志性氨基酸位置。 Ombitasvir (ABT267)对HCV基因型1至6分离株的活性。[1] 鉴于HCV的遗传多样性和NS5A N端区域内的氨基酸多态性程度,我们针对一组未经治疗的基因型1至6分离株评估了ombitasvir的活性,以描述其覆盖范围(表4)。还通过群体测序分析了与欧洲HCV数据库中共识相关的特征性耐药相关氨基酸位置的变异性(41),表4显示了分离物中观察到的多态性。该小组共包括69个基因型1至6的分离株。ombitasvir对66个基因型1至5个分离株的NS5A的EC50范围为0.1至15.1 pM。基因型2a和2b中NS5A第31位氨基酸或基因型4a第28位氨基酸的多态性对ombitasvir的活性没有影响。此外,ombitasvir对基因型2a JFH-1复制子具有活性,EC50为0.82 pM。ombitasir的EC50高于一个具有L28F加M31的基因型2b样本和一个具有A30K变体的基因型3a样本。只有一个基因型6a样本,含有L28,可用于分析。为了更好地代表基因型6a分离物的多态性,将L28F引入可用的基因型6a复制子中。ombitasvir对该基因型6a复制子的L28和F28变体的EC50分别为42 pM和68 pM。 由于其皮摩尔效力和良好的药代动力学,化合物38/Ombitasvir(ABT267)被选为进一步的体外病毒学评估。如表4所示,38不仅对GT1a和GT1b,而且对GT2-GT6都显示出皮摩尔效力。GT1中化合物38的复制子抗性选择实验将28、30和93位的变体确定为主要的抗性相关变体,尽管还观察到其他次要变体(表5)。在GT1a中,变种M28V、L31V和H58D的抗性是38的58至243倍。单一变体M28T、Q30R和Y93C/S的抗性为800至8965倍,而Y93H/N的抗性为38倍,超过40000倍。在GT1b中,体外用38选择的主要变体是Y93H,它具有77倍的抗性。双变体R30Q+Y93H和L31M+Y93H赋予142至284倍的抗性,而所有其他双取代,包括Y93H与28、31或58位的取代结合,赋予38倍以上的抗性。该阻力曲线与BMS-790052有相当大的重叠,尽管在抗折性和所选变体方面存在一些差异。这些对体外选择的高度耐药突变体的观察强调了与DAAs联合治疗的必要性,DAAs通过不同的作用机制抑制病毒复制,以增加耐药性屏障[2]。 |
| 体内研究 (In Vivo) |
在一项为期3天的单一疗法研究中,对12名HCV基因型1感染患者进行了Ombitasvir (ABT267)的体内评估,每天给药5、25、50或200mg。研究中的所有患者都感染了HCV基因型1a,并且通过克隆测序确定在基线时没有预先存在的耐药变异。观察到HCV RNA减少高达3.1 log10IU/ml。在首次给药后48小时,在患者样本中检测到NS5A第28、30或93位的耐药性相关变异。克隆测序分析表明,在3天的单药治疗期间,ombitasvir在很大程度上抑制了野生型病毒,在高于5mg的剂量下,耐药变体M28V也受到了抑制。Ombitasvir在所有剂量下都具有良好的耐受性,没有发生严重或严重的不良事件。这些数据支持ombitasvir与靶向HCV NS3/4A蛋白酶(利托那韦的ABT-450)和HCV NS5B聚合酶(ABT-333,达沙布韦)的抑制剂联合治疗慢性HCV基因型1感染的临床开发。(研究M12-116在ClinicalTrials.gov上注册,注册号为NCT01181427)[1]。
为了评估化合物38/Ombitasvir(ABT267)的安全性、耐受性、药代动力学和抗病毒活性,在HCV GT1感染的初治患者中给药3天,患者每天服用一次38,持续3天,剂量范围为5-200mg。在第3天,各剂量的剂量标准化Cmax和AUC值相似。化合物38的Cmax值范围为5.7至442 ng/mL,各剂量组的半衰期范围为25至32小时。如图3所示,在3天的单一治疗期间,38个剂量组的HCV RNA降低了3.10 log10IU/mL,所有剂量组均观察到近3 log的降低。化合物38在所有剂量下通常耐受良好,没有严重或严重的不良事件,没有临床上显著的实验室异常,也没有受试者停药。大多数不良事件都是轻微的,与剂量无关。[2] 三天单一疗法的药代动力学和抗病毒疗效。[1] 在研究M12-116中,每个剂量组(5、50或200mg每日一次[QD])有6名感染HCV基因型1的初次治疗患者,其中4名患者服用活性药物,2名患者服用匹配的安慰剂3天。在本研究中,18名患者中有16名感染了HCV基因型1a,2名感染了丙型肝炎病毒基因型1b,他们都被随机分配到安慰剂组。由于剂量错误,随机分配到50mg组的两名患者在单药治疗期间实际上接受了25mg。在基线时,18名患者的平均HCV RNA为6.32 log10 IU/ml。在第3天,所有剂量的Ombitasvir(ABT267)剂量标准化最大浓度(Cmax)和浓度-时间曲线下面积(AUC)值相似。Cmax范围为5.7至442 ng/ml,半衰期范围为25.5至32.0小时。图3显示了12名服用ombitasvir的HCV感染患者的个体HCV RNA病毒载量下降。在所有研究剂量中都观察到了类似的强效抗病毒反应,在3天的单药治疗期间,HCV RNA的平均最大减少量高达3.10 log10 IU/ml,而在研究的安慰剂组中,HCV RNA平均减少量为0.15 log10 IU/ml。总的来说,ombitasvir在所有给药剂量下都具有良好的耐受性。大多数不良事件是轻微的、自限性的、持续时间短的,被认为与ombitasvir无关或可能无关。不良事件没有剂量反应模式。未报告死亡或严重不良事件。 体内耐药性发展评估。[1] 在本研究中,对所有接受Ombitasvir (ABT267)治疗的患者进行了NS5A编码区的克隆测序和表型分析,这些患者在基线、第3天和/或第6天的病毒滴度≥1000 IU/ml(图3和表5)。在3个剂量组中,所有12名服用Ombitasvir(ABT267)的患者均感染了基因型1a病毒,通过克隆序列分析,在基线时未在任何患者中检测到耐药相关氨基酸位置28、30、31、58或93的变异。 在5mg QD剂量组中,4名患者中有2名在第3天和第6天的HCV RNA病毒载量均≥1000IU/ml。患者1在第3天主要为M28V,但在第6天为M28T、M28V和Q30R的混合物。患者2在第3天和第6天主要携带野生型病毒,M28V和Q30R为次要变异。在接受25mg剂量的2名患者中,1名(患者3)在第3天和第6天的HCV RNA病毒载量≥1000IU/ml。在该患者的样本中,在第3天观察到M28T、M28V、Q30R和Y93C;然而,在第6天不再检测到M28V变体。与这些测序观察结果一致,在表型测定中,来自患者1、2和3的第6天样本分别对<强>奥比他韦(ABT267)产生98倍、4倍和36倍的耐药性。 在接受50mg剂量的Ombitasvir (ABT267)的2名患者中,M28T在第3天和第6天都是患者4的主要变异株,尽管也检测到了M28V、Q30E、Q30R和Y93C。50mg剂量组的患者5在开始服用ombitasvir后36小时出现HCV RNA病毒载量最低点,仅比基线值降低1.52 log10IU/ml。尽管在基线时没有检测到预先存在的耐药性相关变异,但患者5在第3天和第6天出现了复杂的变异混合物。Y93S和Q30L加Y93H是主要的变异,而Y93H和Q30L-加Y93S也被检测为次要变异。对HCV基因型1a-H77复制子中这些变体的体外分析(表2)表明,Q30L加Y93H(416倍)或Q30L加Y93S(218倍)赋予的耐药性低于单独由Y93H或Y93S赋予的耐药性。与耐药变异的存在一致,在表型分析中,患者4和5的第6天样本分别对ombitasvir产生353倍和406倍的耐药性。 在200mg剂量组中,4名服用活性药物的患者中有3名在第3天和/或第6天的病毒载量≥1000IU/ml HCV RNA。在第3天和第6天,M28T和Q30R是所有3名患者的主要变异,而Y93C和Y93H是次要变异。与耐药变体的存在一致,在表型分析中,来自患者6、7和8的第6天样本对奥美他韦(ABT267)的耐药性为927至2238倍。 第3天或第6天的临床样本通常含有NS5A变体的混合物(包括野生型)。这些变体中的每一个都可能具有不同的复制适应性和对Ombitasvir (ABT267)的可变易感性,因此对临床分离株的EC50反映了准物种中存在的多种变体。在许多情况下,携带单个或多个确定的耐药变体的参考复制子的EC50与临床分离株的EC50不同。 |
| 酶活实验 |
先前描述的技术用于量化不同氨基酸变体如何影响 HCV 复制子细胞培养测定中的抑制剂活性。简而言之,使用 Change-IT 多重突变定点诱变试剂盒,将 NS5A 中的抗性相关变体分别引入基因型 1a-H77 或 1b-Con1 或嵌合体之一基因型 2 至 6 个复制子。序列分析验证变体的存在后,将质粒线性化,并使用 TranscriptAid T7 高产转录试剂盒 提取 HCV 亚基因组 RNA。通过电穿孔,在临时实验中用含有变体的复制子 RNA 转染 Huh-7 细胞系。如前面部分所述,计算了 EC50。
复制子细胞系和体外活性测定。[1] 通过测量萤光素酶报告基因的活性,在含有5%FBS的DMEM中测定了Ombitasvir (ABT267)对HCV复制的抑制作用,该DMEM含有或不含有40%的人血浆。细胞在ombitasvir存在下孵育3天,随后根据制造商的说明裂解和处理。使用Victor II光度计测量细胞中的萤光素酶活性。使用非线性回归曲线拟合4参数逻辑斯谛方程和GraphPad Prism 4软件计算50%有效浓度(EC50)。通过3-[4,5-二甲基噻唑-2-基]-2,5-二苯基溴化四唑(5mg/ml)比色法测定ombitasvir的细胞毒性。使用非线性回归曲线拟合4参数逻辑方程和GraphPad Prism 4软件,根据光密度数据计算50%细胞毒性浓度(CC50)。南方研究所使用不含萤光素酶报告子的亚基因组复制子,通过定量RT-PCR(qRT-PCR)分析评估了ombitasvir对基因型2a JFH-1的活性。 临床分离株NS5A基因的表型。[1] 构建了一个基于基因型1b-Con1 HCV复制子的穿梭载体盒,带有萤光素酶报告基因,但没有Neo基因,用于评估来自感染HCV基因型1至6的个体的NS5A基因的表型。将NotI限制性位点克隆到NS4B 3′端NS5A上游90个核苷酸的1b-Con1亚基因组复制子载体中,并在NS5A氨基酸413密码子后克隆ClaI位点。基因型1感染患者的NS5A区域插入NotI和ClaI限制性位点之间。使用具有NotI和BlpI限制性位点的1b-Con1穿梭载体(如前一节所述)来评估Ombitasvir (ABT267)抑制非基因型1 HCV中包含氨基酸1至214的NS5A区域的能力。扩增临床样本中的NS5A基因并将其连接到穿梭载体中。在瞬时测定中,将来自每个临床样本的含有NS5A基因的HCV亚基因组复制子RNA通过电穿孔转染到Huh-7衍生的细胞系中。细胞在ombitasvir存在下孵育4天。测量细胞中的萤光素酶活性,并如上所述计算EC50。 体外耐药性分析。[1] 为了表征对Ombitasvir (ABT267)敏感性降低的复制子变体,在上述含有HCV复制子的嵌合基因型1至6稳定细胞系中进行了耐药性选择。复制子细胞(5×104至1×106)被放置在150 mm的细胞培养板上,并在G418(400μg/ml)和ombitasvir的存在下生长,其浓度比相应细胞系的EC50高10倍、50倍、100倍或1000倍。经过3周的治疗,大多数复制子细胞中的复制子RNA被清除,因此无法在含G418的培养基中存活。含有抗性复制子变体的细胞存活下来并形成集落,每个集落都被挑选并进一步扩增。为了表征抗性复制子变体的基因型,将扩增的菌落在CellsDirect再悬浮和裂解缓冲液中裂解,得到总RNA。通过RT-PCR扩增NS5A编码区,使用ABI Prism染料终止子循环测序试剂盒对扩增的样品进行测序,并在Applied Biosystems 3100遗传分析仪上进行分析。 |
| 细胞实验 |
创建了一个基于基因型 1b-Con1 HCV 复制子的穿梭载体盒,该载体缺乏 Neo 基因,但含有荧光素酶报告基因,用于评估感染 HCV 基因型 1 至 6 的患者的 NS5A 基因表型。 NS5A 上游 3' 端有 90 个核苷酸NS4B(NotI 限制性位点)被克隆到 1b-Con1 亚基因组复制子载体中,ClaI 位点被克隆到 NS5A 氨基酸 413 密码子之后。 NotI 和 ClaI 限制性位点与基因型 1 感染患者的 NS5A 区域交叉。使用具有 NotI 和 BlpI 限制性位点的 1b-Con1 穿梭载体(在上一节中讨论)评估了奥比他韦抑制非基因型 1 HCV NS5A 区域(包含氨基酸 1 至 214)的能力。使用临床样本将 NS5A 基因的扩增副本连接到穿梭载体中。一项瞬时测定涉及将每个临床样本的含有 NS5A 基因的 HCV 亚基因组复制子 RNA 电穿孔到源自 Huh-7 的细胞系中。奥比他韦在细胞培养的四天内一直存在。按照前面提到的程序,测定细胞中的荧光素酶活性。
GT1a复制子构建体(GenBank登录号NC004102)包含1a-H77的5′非翻译区(NTR),随后是萤火虫荧光素酶报告基因和新霉素磷酸转移酶(Neo)基因,它们共同构成了双顺反子复制子构建物的第一顺反子。随后是EMCV IRES,然后是第二个包含1a-H77 NS3-NS5B编码区的顺反子,该编码区具有适应性突变E1202G、K1691R、K2040R和S2204I,最后是1a-H77 3′NTR。GT1b-Con1复制子构建体(GenBank登录号AJ238799)包含1b-Con-1的5′非翻译区(NTR),随后是萤火虫荧光素酶报告基因和新霉素磷酸转移酶(Neo)基因,它们共同构成了双顺反子复制子构建物的第一顺反子。随后是EMCV IRES和第二个含有1b-Con1 NS3-NS5B编码区的顺反子,该编码区具有适应性突变K1609E、K1846T和Y3005C,最后是1b-Con3′NTR。此外,1b-Con1复制子构建体在HCV 5′NTR和萤火虫荧光素酶基因之间含有脊髓灰质炎病毒IRES。为了评估化合物抑制NS5A对抗非GT1 HCV的能力,构建了一个1b-Con1复制子穿梭载体,该载体在NS4B的C末端NS5A上游和NS5A氨基酸214之后含有限制性位点。生成了六个嵌合亚基因组复制子细胞系,用于评估化合物的活性。用于产生嵌合复制子的NS5A区域来源于基因型2a、2b、3a、4a、5a和6a HCV感染患者血清。对来自这些患者血清样品中的每一个的病毒RNA进行RT-PCR,以产生编码NS5A的前214个氨基酸的DNA片段。将该片段连接到复制子穿梭载体中,并在体外转录为复制子RNA。将该RNA引入人肝癌细胞(Huh-7)以创建稳定的复制子细胞系。通过测量萤光素酶信号的减少来确定化合物对HCV复制的抑制作用(在40%HP存在或不存在的情况下)。计算每种化合物浓度对HCV复制子复制的抑制百分比,并使用GraphPad Prism 4/5软件中的四参数逻辑斯谛方程的非线性回归曲线拟合计算EC50值。 前面描述了描述在HCV复制子细胞培养试验中测量单个氨基酸变体对抑制剂活性影响的方法。简而言之,NS5A中的抗性相关变体分别被引入GT 1a-H77、GT 1b-Con1或嵌合复制子中。在瞬时检测中,通过电穿孔将含有该变体的复制子转染到Huh-7衍生的细胞系中。对于每种化合物浓度,计算由萤光素酶信号降低确定的HCV复制子复制的抑制百分比,并如上所述计算EC50值。使用以下方程式计算复制能力作为野生型复制的百分比,100×{(变体4天萤光素酶计数/野生型4天萤光素酶计数)/(变体4小时萤光素酶数/野生型4h萤光素酶计数)}[2]。 |
| 动物实验 |
Clinical study design. Study M12-116 (ClinicalTrials.gov registration no. NCT01181427) was the first study to evaluate the pharmacokinetics, safety, tolerability, antiviral activity, and resistance of ombitasvir in HCV-infected treatment-naive adults. All of the patients provided written informed consent. The study was performed in accordance with Good Clinical Practice guidelines and the principles of the Declaration of Helsinki, and the study protocol was approved by the relevant institutional review boards and regulatory agencies. Inclusion criteria included chronic HCV genotype 1 infection for at least 6 months prior to study enrollment, plasma HCV RNA level of >100,000 IU/ml at screening, and a liver biopsy within the past 3 years with histology consistent with HCV-related inflammation and fibrosis but no evidence of cirrhosis. Exclusion criteria included positive antibodies for hepatitis A or B virus or human immunodeficiency virus type 1 (HIV-1) or a history of clinically significant comorbidities. The primary endpoint was the maximum change from baseline in HCV RNA. The patients in the ombitasvir dose groups were enrolled sequentially, and within each group, patients were randomized (2:1) to either ombitasvir or placebo and treated under nonfasting conditions for 3 days while confined to the study site. The 200-mg dose group received a different formulation with higher bioavailability. Patients who received at least one dose of ombitasvir or placebo were provided the option to receive treatment with pegIFN/RBV for approximately 48 weeks once treatment with ombitasvir was completed. HCV RNA was measured using the Roche COBAS TaqMan HCV Test v2.0 real-time reverse transcriptase PCR assay (with a lower limit of quantification of 25 IU/ml and a lower limit of detection of 10 IU/ml). The virologic response was assessed as HCV RNA decrease from baseline in log10 IU/ml[1].
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Ombitasvir reaches peak plasma concentration 5 hours after administration. It has an absolute bioavailability of 48%. Taking ombitasvir with high or normal fat meals increases exposure by 1.76 or 1.82 fold respectively. Ombitasvir is mainly excreted in the feces (90.2%) with very little excreted in the urine (1.91%). 87.8% and 0.03% of the dose excreted in the feces and urine respectively is present as the parent compound. Ombitasvir has a volume of distribution at steady state of 173 liters. Clearance of Ombitasvir has not been determined. Metabolism / Metabolites Ombitasvir is mainly metabolized by amide hydrolysis followed by CYP2C8-mediated oxidative metabolism. Biological Half-Life Ombitasvir has a half life of elimination of 21-25 hours Compound 38/Ombitasvir (ABT267) demonstrated high stability in RLM and long half-lives in rat (6, 4, and 9 h, respectively), although they suffered from low oral bioavailability. However, compound 38 demonstrated significantly higher oral bioavailability and exposures in the dog compared to compound 32 and 33. Unlike compounds 13 and 16, compound 38 also demonstrated a reasonable half-life with low clearance and moderate oral bioavailability in monkey. The tert-butylglycine analogues 40 and 41 showed comparable rat PK to the valine analogues 38 and 39, including low oral bioavailability, likely a result of their high lipophilicity (log D = 5.5) and low aqueous solubility. [2] |
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation Ombitasvir has not been studied in nursing mothers being treated for hepatitis C infection. Because it is 99.9% bound to maternal plasma proteins, amounts in breastmilk are likely to be very low. Some sources recommend against breastfeeding when ombitasvir is used with ribavirin. Ritonavir used as a booster has been studied in several studies of breastfeeding mothers. It is excreted into milk in measurable concentrations and low levels can be found in the blood of some breastfed infants. No reports of adverse reactions in breastfed infants have been reported. For more information, refer to the LactMed record on ritonavir. Hepatitis C is not transmitted through breastmilk and breastmilk has been shown to inactivate hepatitis C virus (HCV). However, the Centers for Disease Control recommends that mothers with HCV infection should consider abstaining from breastfeeding if their nipples are cracked or bleeding. It is not clear if this warning would apply to mothers who are being treated for hepatitis C. Infants born to mothers with HCV infection should be tested for HCV infection; because maternal antibody is present for the first 18 months of life and before the infant mounts an immunologic response, nucleic acid testing is recommended. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Ombitasvir is 99.9% bound to human plasma proteins. |
| 参考文献 | |
| 其他信息 |
Pharmacodynamics
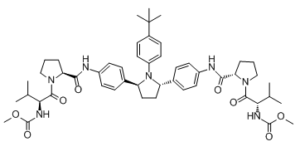
Ombitasvir is classified as a direct acting antiviral and acts against HCV to inhibit viral replication. Ombitasvir is a dipeptide derivative which is used which is in combination with dasabuvir sodium hydrate, paritaprevir and ritonavir (under the trade name Viekira Pak) for treatment of chronic hepatitis C virus genotype 1 infection as well as cirrhosis of the liver. It has a role as an antiviral drug and a hepatitis C virus nonstructural protein 5A inhibitor. It is a member of pyrrolidines, a carbamate ester, an aromatic amide and a dipeptide. It is functionally related to a Val-Pro. Ombitasvir is a direct acting antiviral medication used as part of combination therapy to treat chronic Hepatitis C, an infectious liver disease caused by infection with Hepatitis C Virus (HCV). HCV is a single-stranded RNA virus that is categorized into nine distinct genotypes, with genotype 1 being the most common in the United States, and affecting 72% of all chronic HCV patients. Treatment options for chronic Hepatitis C have advanced significantly since 2011, with the development of Direct Acting Antivirals (DAAs) such as Ombitasvir. Ombitasvir is an inhibitor of NS5A, a protein essential for viral replication and virion assembly. The barrier for develoment of resistance to NS5A inhibitors is lower than that of NS5B inhibitors, another class of DAAs. In a joint recommendation published in 2016, the American Association for the Study of Liver Diseases (AASLD) and the Infectious Diseases Society of America (IDSA) recommend Ombitasvir as a first line therapy option when used in combination with other antivirals for genotypes 1a, 1b, and 4. Depending on the genotype, Ombitasvir is often used in combination with other antivirals such as [DB09183], [DB09297], [DB00503], and [DB00811] with the intent to cure, or achieve a sustained virologic response (SVR), after 12 weeks of daily therapy. SVR and eradication of HCV infection is associated with significant long-term health benefits including reduced liver-related damage, improved quality of life, reduced incidence of Hepatocellular Carcinoma, and reduced all-cause mortality. Treatment with direct acting antivirals such as Ombitasvir is associated with very minimal side effects, with the most common being headache and fatigue. Lack of significant side effects and short duration of therapy is a considerable advantage over older interferon-based regimens, which were limited by infusion site reactions, reduced blood count, and neuropsychiatric effects. Ombutasvir first came on the market as a fixed-dose combination product with [DB09183], [DB09297], and [DB00503] as the FDA-approved product Viekira Pak. First approved in December 2014, Viekira Pak is indicated for the treatment of HCV genotype 1b without cirrhosis or with compensated cirrhosis, and when combined with Ribavirin for the treatment of HCV genotype 1a without cirrhosis or with compensated cirrhosis. Ombutasvir is also available as a fixed-dose combination product with [DB09297] and [DB00503] as the FDA- and Health Canada-approved product Technivie. First approved in July 2015, Technivie is indicated in combination with Ribavirin for the treatment of patients with genotype 4 chronic hepatitis C virus (HCV) infection without cirrhosis or with compensated cirrhosis. In Canada, Ombutasvir is also available as a fixed-dose combination product with [DB09183], [DB09297], and [DB00503] as the Health Canada-approved, commercially available product Holkira Pak. First approved in January 2015, Holkira Pak is indicated for the treatment of HCV genotype 1b with or without cirrhosis, and when combined with [DB00811] for the treatment of HCV genotype 1a with or without cirrhosis. Ombitasvir is a Hepatitis C Virus NS5A Inhibitor. The mechanism of action of ombitasvir is as an UGT1A1 Inhibitor. Ombitasvir is an orally available inhibitor of the hepatitis C virus (HCV) non-structural protein 5A (NS5A) replication complex, with potential activity against HCV. Upon oral administration and after intracellular uptake, ombitasvir binds to and blocks the activity of the NS5A protein. This results in the disruption of the viral RNA replication complex, blockage of HCV RNA production, and inhibition of viral replication. NS5A, a zinc-binding and proline-rich hydrophilic phosphoprotein, plays a crucial role in HCV RNA replication. HCV is a small, enveloped, single-stranded RNA virus belonging to the Flaviviridae family; HCV infection is associated with the development of hepatocellular carcinoma (HCC). OMBITASVIR is a small molecule drug with a maximum clinical trial phase of IV (across all indications) that was first approved in 2014 and is indicated for hepatitis c virus infection and chronic hepatitis c virus infection and has 1 investigational indication. Ombitasvir (ABT267) is a hepatitis C virus (HCV) NS5A inhibitor with picomolar potency, pan-genotypic activity, and 50% effective concentrations (EC50s) of 0.82 to 19.3 pM against HCV genotypes 1 to 5 and 366 pM against genotype 6a. Ombitasvir retained these levels of potency against a panel of 69 genotype 1 to 6 chimeric replicons containing the NS5A gene derived from HCV-infected patients, despite the existence of natural sequence diversity within NS5A. In vitro resistance selection identified variants that conferred resistance to ombitasvir in the HCV NS5A gene at amino acid positions 28, 30, 31, 58, and 93 in genotypes 1 to 6. Ombitasvir was evaluated in vivo in a 3-day monotherapy study in 12 HCV genotype 1-infected patients at 5, 25, 50, or 200 mg dosed once daily. All patients in the study were HCV genotype 1a infected and were without preexisting resistant variants at baseline as determined by clonal sequencing. Decreases in HCV RNA up to 3.1 log10 IU/ml were observed. Resistance-associated variants at position 28, 30, or 93 in NS5A were detected in patient samples 48 hours after the first dose. Clonal sequencing analysis indicated that wild-type virus was largely suppressed by ombitasvir during 3-day monotherapy, and at doses higher than 5 mg, resistant variant M28V was also suppressed. Ombitasvir was well tolerated at all doses, and there were no serious or severe adverse events. These data support clinical development of ombitasvir in combination with inhibitors targeting HCV NS3/4A protease (ABT-450 with ritonavir) and HCV NS5B polymerase (ABT-333, dasabuvir) for the treatment of chronic HCV genotype 1 infection. (Study M12-116 is registered at ClinicalTrials.gov under registration no. NCT01181427.).[1] Ombitasvir (ABT267) was evaluated in the dose-ranging clinical study M12-116, in which the maximum change from baseline in HCV RNA was evaluated along with a thorough resistance analysis of samples obtained within the first few days after initiation of ombitasvir dosing. In M12-116, 12 treatment-naive patients infected with HCV genotype 1 were administered 5, 25, 50, or 200 mg once daily of ombitasvir for 3 days, followed by an optional regimen of pegIFN and RBV for 48 weeks. All ombitasvir-treated patients were genotype 1a infected, and the mean maximum declines in HCV RNA observed across the doses studied were up to 3.10 log10 IU/ml during the 3-day monotherapy. None of the patients had preexisting resistance-conferring variants at signature amino acid position 28, 30, 31, 58, or 93 in NS5A detectable by clonal sequencing. Postbaseline samples from 8 patients were available for resistance analyses. Variants M28T, M28V, and Q30R in NS5A were the predominant treatment-emergent variants, while Y93C and Y93H were detected as minor variants. On day 3 of treatment or day 6 (48 h posttreatment), greater than 90% of the clones from each of the patient samples contained variants at signature resistance-conferring amino acid positions, indicating that in most patients, the wild-type virus had been suppressed. At the 5-mg dose, M28V was detected in two patients at day 3 and day 6; however, at higher doses where the Cmax values also increased, M28T or Q30R was the predominant variant. This is consistent with the observation in vitro that the M28V variant, which confers 58-fold resistance to ombitasvir, can be suppressed at higher doses of ombitasvir. In line with the genotypic observations, phenotypic analysis indicated that samples from patients in the 5-mg and 25-mg dose groups conferred 1- to <100-fold resistance to ombitasvir, while most samples from patients receiving 50 or 200 mg of ombitasvir conferred >500-fold resistance to ombitasvir. Although ombitasvir demonstrated similarly robust antiviral responses across all doses studied, the in vivo resistance profile suggest a benefit of using a dose higher than 5 mg of ombitasvir in order to suppress prevalent preexisting variants such as M28V in genotype 1a or Y93H in genotype 1b. The in vitro profile Ombitasvir (ABT267) and the results in the 3-day monotherapy study M12-116 provided the basis for investigating the combination of ombitasvir with the NS3/4A protease inhibitor ABT-450 and nonnucleoside NS5B polymerase inhibitor dasabuvir (ABT-333) in treatment of chronic genotype 1 HCV infection. The combination of these 3 DAAs provides a barrier to resistance in patients, as evidenced by the high SVR rate in the six phase 3 clinical trials using an interferon-free combination of ombitasvir/ABT-450/ritonavir and dasabuvir with or without ribavirin.[1] We describe here N-phenylpyrrolidine-based inhibitors of HCV NS5A with excellent potency, metabolic stability, and pharmacokinetics. Compounds with 2S,5S stereochemistry at the pyrrolidine ring provided improved genotype 1 (GT1) potency compared to the 2R,5R analogues. Furthermore, the attachment of substituents at the 4-position of the central N-phenyl group resulted in compounds with improved potency. Substitution with tert-butyl, as in compound 38/Ombitasvir (ABT267), provided compounds with low-picomolar EC50 values and superior pharmacokinetics. It was discovered that compound 38 was a pan-genotypic HCV inhibitor, with an EC50 range of 1.7-19.3 pM against GT1a, -1b, -2a, -2b, -3a, -4a, and -5a and 366 pM against GT6a. Compound 38 decreased HCV RNA up to 3.10 log10 IU/mL during 3-day monotherapy in treatment-naive HCV GT1-infected subjects and is currently in phase 3 clinical trials in combination with an NS3 protease inhibitor with ritonavir (r) (ABT-450/r) and an NS5B non-nucleoside polymerase inhibitor (ABT-333), with and without ribavirin. [2] There is a tremendous unmet medical need to discover new treatments for HCV infection with improved efficacy, tolerability, and convenience relative to the current standard of care. The addition of inhibitors of HCV NS5A to the arsenal of DAAs that are available for use in combination therapy holds significant promise for delivering such therapies. We discovered compound 38/Ombitasvir (ABT267), based on a chiral pyrrolidine core, which demonstrates picomolar potency against GT1a and GT1b. Compound 38 is a pan-genotypic HCV inhibitor, with an EC50 range of 1.7–19.3 pM against genotypes 1a, 1b, 2a, 2b, 3a, 4a, and 5a and 366 pM against genotype 6a. Subsequent to its discovery, compound 38 has demonstrated excellent human pharmacokinetics with a long 25–32 h half-life, consistent with once-daily dosing. Compound 38 decreased HCV RNA up to 3.10 log10 IU/mL during 3-day monotherapy in treatment-naive HCV GT1-infected subjects, and it is currently undergoing phase 3 clinical trials in combination with an NS3 protease inhibitor and an NS5B non-nucleoside polymerase inhibitor, with and without ribavirin.[2] |
| 分子式 |
C50H67N7O8
|
|
|---|---|---|
| 分子量 |
894.11
|
|
| 精确质量 |
893.505
|
|
| 元素分析 |
C, 67.17; H, 7.55; N, 10.97; O, 14.32
|
|
| CAS号 |
1258226-87-7
|
|
| 相关CAS号 |
|
|
| PubChem CID |
54767916
|
|
| 外观&性状 |
White to light yellow solid powder
|
|
| 密度 |
1.2±0.1 g/cm3
|
|
| 沸点 |
1065.6±65.0 °C at 760 mmHg
|
|
| 闪点 |
598.2±34.3 °C
|
|
| 蒸汽压 |
0.0±0.3 mmHg at 25°C
|
|
| 折射率 |
1.595
|
|
| LogP |
6.29
|
|
| tPSA |
178.72
|
|
| 氢键供体(HBD)数目 |
4
|
|
| 氢键受体(HBA)数目 |
9
|
|
| 可旋转键数目(RBC) |
16
|
|
| 重原子数目 |
65
|
|
| 分子复杂度/Complexity |
1540
|
|
| 定义原子立体中心数目 |
6
|
|
| SMILES |
CC(C)(C)C(C=C1)=CC=C1N2[C@H](C3=CC=C(NC([C@H]4N(C([C@@H](NC(OC)=O)C(C)C)=O)CCC4)=O)C=C3)CC[C@H]2C5=CC=C(NC([C@@H]6CCCN6C([C@@H](NC(OC)=O)C(C)C)=O)=O)C=C5
|
|
| InChi Key |
PIDFDZJZLOTZTM-KHVQSSSXSA-N
|
|
| InChi Code |
InChI=1S/C50H67N7O8/c1-30(2)42(53-48(62)64-8)46(60)55-28-10-12-40(55)44(58)51-35-20-14-32(15-21-35)38-26-27-39(57(38)37-24-18-34(19-25-37)50(5,6)7)33-16-22-36(23-17-33)52-45(59)41-13-11-29-56(41)47(61)43(31(3)4)54-49(63)65-9/h14-25,30-31,38-43H,10-13,26-29H2,1-9H3,(H,51,58)(H,52,59)(H,53,62)(H,54,63)/t38-,39-,40-,41-,42-,43-/m0/s1
|
|
| 化学名 |
methyl N-[(2S)-1-[(2S)-2-[[4-[(2S,5S)-1-(4-tert-butylphenyl)-5-[4-[[(2S)-1-[(2S)-2-(methoxycarbonylamino)-3-methylbutanoyl]pyrrolidine-2-carbonyl]amino]phenyl]pyrrolidin-2-yl]phenyl]carbamoyl]pyrrolidin-1-yl]-3-methyl-1-oxobutan-2-yl]carbamate
|
|
| 别名 |
Viekira Pak (trade name); ABT-267; Ombitasvir; 1258226-87-7; Ombitasvir [INN]; CHEBI:85183; Ombitasvir [USAN:INN]; ABT 267; UNII-2302768XJ8; Ombitasvir(ABT-267); ABT267; CHEBI:85183; 2302768XJ8; ABT267; ABT 267
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (2.80 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (2.80 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL 澄清 DMSO 储备液加入到 900 μL 玉米油中并混合均匀。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.1184 mL | 5.5922 mL | 11.1843 mL | |
| 5 mM | 0.2237 mL | 1.1184 mL | 2.2369 mL | |
| 10 mM | 0.1118 mL | 0.5592 mL | 1.1184 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
A Follow up Study Designed to Obtain Long Term Data on Participants Who Either Achieved a Sustained Virologic Response or Did Not Achieve a Sustained Virologic Response in an AbbVie Sponsored Hepatitis C Study
CTID: NCT01773070
Phase: Phase 3 Status: Completed
Date: 2017-12-06
Alignment of amino acids 1 to 100 of NS5A in the replicon cell lines. Amino acid changes relative to the 1b-Con1 sequence are indicated. Amino acids within each genotype where variants resistant to ombitasvir were selected are highlighted in gray.Antimicrob Agents Chemother.2015 Feb;59(2):979-87. |
|---|
HCV RNA viral load during 3-day monotherapy with ombitasvir in HCV genotype 1-infected treatment-naive patients.Antimicrob Agents Chemother.2015 Feb;59(2):979-87. |