| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg | |||
| Other Sizes |
| 靶点 |
FP ( Ki = 790 nM ); TP Receptor ( Ki = 2400 nM ); EP4 ( Ki = 1.3 nM ); EP3 ( Ki = 30 nM )
|
|---|---|
| 体外研究 (In Vitro) |
ONO-AE3-208以剂量依赖性方式抑制体外细胞侵袭和迁移,而不影响细胞增殖[2]。 ONO-AE3-208 在 EET 合成抑制剂 MS-PPOH 存在的情况下消除 CTGF。花生四烯酸 (AA) 会引起所附着的 Af-Art 的剂量依赖性扩张,这种效应会被 ONO-AE3-208 阻断[3]。
EP4拮抗剂ONO-AE3-208抑制前列腺癌细胞的侵袭和迁移而不影响细胞增殖[3]。 通过细胞增殖实验,观察ONO-AE3-208对PC3、LNCaP、LNCaP/mock、LNCaP/EP4+细胞增殖的影响。10µmol/L的ONO-AE3-208没有改变这些细胞的增殖率,尽管细胞表达了EP4。 |
| 体内研究 (In Vivo) |
ONO-AE3-208 抑制小鼠 PC3 细胞的体内骨转移[2]。与ONO-AE3-208治疗组相比,对照组的光子肿瘤负荷以时间依赖性方式显着增加。前者的转移形成率明显高于后者。 ONO-AE3-208 治疗动物中转移形成的中位时间为 29 天,而对照组为 21 天[4]。
EP4抑制剂ONO-AE3-208可减轻链脲佐菌素糖尿病eNOS敲除小鼠的蛋白尿。 ONO-AE3-208可减少db/db小鼠的蛋白尿和系膜基质积聚。 ONO-AE3-208减轻亚全肾切除大鼠的肾损伤。[5] Connecting tubule glomerular feedback (CTGF) 实验Protocol[2] 1)实验#2和#3的时间控制:将CNT内的管腔NaCl从10 mmol/ l增加到80 mmol/ l,产生3条连续的浓度-响应曲线。 2) EP4拮抗剂ONO-AE3-208对CTGF的影响:CNT内管腔NaCl从10 mmol/L增加到80 mmol/L,形成3条连续的浓度-响应曲线。第二和第三条曲线分别添加EET合成抑制剂MS-PPOH(10−6 mol/L),第三条曲线添加EP4拮抗剂ONO-AE3-208(10−7 mol/L)。ONO-AE3-208的浓度是EP4受体Ki的77倍,EP2受体Ki的至少100倍。 3) EP4拮抗剂L161982对CTGF的影响:本实验与#2相似,但用EP4拮抗剂L161982(10−5 mol/L)代替ONO-AE3-208。L161982的浓度是EP4受体Ki的312倍,EP2受体Ki的6倍。 4)内皮破坏对CTGF的影响:CNT中NaCl从10 mmol/L增加到80 mmol/L,可诱导CTGF。将山羊抗血管性血液病因子抗人抗体(14.29 mg/ml稀释1:1000)加2%豚鼠补体灌注到Af-Art腔内10分钟,冲洗20分钟,再次诱导CTGF。为了确认内皮的完全功能去除,我们在Af-Art的管腔中添加了10 - 5 mol/L的乙酰胆碱,我们反复证明该浓度足以使Af-Art扩张。 5)外源性花生四烯酸(AA)对碳纳米管的影响:在NE预缩Af-Art后,在没有NaCl的情况下,将AA浓度从10−7到10−5 mol/L添加到碳纳米管的管腔中。在实验结束时,我们去除AA并将CNT管腔灌注液切换到80 mmol/L NaCl。 6) EP4拮抗剂对外源性AA诱导的CTGF的影响:本实验与#5相似,不同之处是将MS-PPOH添加到CNT的管腔中,并将ONO-AE3-208添加到管腔中。 在所有实验中,Af-Art直径都是在间隔3-5 μm的三个位点对NE的最大响应区域测量的,并表示为这三个测量值的平均值。用摄像机每隔5秒记录直径,用装有Metavue图像分析软件的计算机测量直径。 |
| 细胞实验 |
细胞增殖试验[3]
采用细胞计数试剂盒-8 (CCK8)检测ONO-AE3-208对细胞增殖的影响。5 × 10~3细胞(PC3细胞)和1 × 104细胞(LNCaP、LNCaP/mock和LNCaP/EP4+细胞)接种于96孔板。然后,每隔24 h,用10 μl CCK8试剂在37°温度下染色2 h,连续72 h,用自动板仪在450 nm处定量染色反应。每个实验重复三次,独立进行三次。 入侵检测[3] 采用BD BioCoat Matrigel侵袭室检测前列腺癌细胞的侵袭活性。用PBS洗涤细胞,在不含FBS的培养基中重悬,浓度为3 × 104个细胞/ml。上腔涂布基质上加入细胞悬液500 μl,下腔中加入含1% FBS的培养基750 μl。在5% CO2培养箱中37°C孵育24 h后,用棉签擦拭滤膜上表面的细胞。滤网用70%乙醇固定,苏木精染色。在显微镜下随机选择6个视野对染色细胞进行计数。至少分析了来自三个不同实验的三个腔室。 愈合试验[3] 创面愈合实验如前所述进行。6孔培养皿中未融合的PC3、LNCaP、LNCaP/mock和LNCaP/EP4+细胞用塑料吸管尖划伤培养24 h。通过图像J (http://rsbweb.nih.gov/ij/)测量“创面”(划伤区域)的宽度,创面愈合比例按以下公式计算:100% - (24 h后的宽度/开始时的宽度)× 100%。每个实验重复三次,独立进行三次。 |
| 动物实验 |
Animal model 1: Induction of colitis.[1]
DSS of the average molecular weight of 5,000 was administered to 8-week-old mice for 7 days at either 3% (low dose) or 7% (high dose) concentration in the drinking water. The addition of DSS or any drugs mentioned below to the drinking water did not affect water consumption of mice. Indomethacin also was added to the drinking water at a dose of 4 mg/kg/day and administered to the animals during the entire experimental period. This dose of indomethacin was reported to inhibit PGE2 production in rats and mice in vivo. An EP4 antagonist, ONO-AE3-208, 4-{4-Cyano-2-[2-(4-fluoronaphthalen-1-yl) propionylamino] phenyl} butyric acid (AE3-208), and an EP4 agonist, ONO-AE1-734, methyl-7-[(1R, 2R, 3R)-3-hydroxy-2-[(E)-(3S)-3-hydroxy-4-(m-methoxymethylphenyl)-1-butenyl]-5-oxocyclopenthl]-5-thiaheptanoate (AE1-734), were used. The Ki values of ONO-AE3-208 obtained by competition-binding isotherms to displace the radioligand binding to the respective prostanoid receptor are 1.3, 30, 790, 2,400 nM for EP4, EP3, FP, and TP, respectively, and more than 10,000 nM for the other prostanoid receptors. The Ki values of AE1-734 are 0.7, 56, and 620 nM for EP4, EP3, and EP2, respectively, and more than 10,000 nM for the rest of the prostanoid receptors. ONO-AE3-208 was administered (10 mg/kg/day) orally in the drinking water. When this compound was administered orally at 10 mg/kg as a bolus, a peak plasma concentration of 677 ng/ml was attained in 0.25 hours after the administration with 18% of bioavailability. The plasma half-life of this compound measured in an experiment of intravenous injection was 0.2 hours. AE1-734 was administered subcutaneously twice a day (0.1 mg/kg/each) from 1 day before the DSS treatment until the end of experiment. When AE1-734 was injected subcutaneously at this dose, the peak plasma concentration of 100 ng/ml was attained at 10 minutes after the injection with more than 70% for bioavailability. The plasma concentration declined with a half-life of 30 minutes. To evaluate mucosal integrity, the FITC-dextran assay was used. Wild-type C57BL/6 mice were administered with either ONO-AE3-208 or vehicle in the drinking water. After 1 day, both groups of mice were fed with 3% DSS in the drinking water in the continued presence or absence of ONO-AE3-208, and 24 hours later, 200 μl of FITC-dextran (average molecular weight, 4,400) (2 mg/ml in saline) was administered orally. Serum concentration was determined 4 hours after the administration of FITC-dextran. EP4–/– mice and their wild-type control mice were similarly treated with 3% DSS and administered with FITC-dextran. The colon was snap-frozen and cryostat sections of 10-μm thickness were used for fluorescent microscopic analysis. Animal model 2: Bone Metastasis Animal Model and Bioluminescent Imaging[3] To establish bone metastasis, 1 × 105 PC3/Luc cells suspended in 100 µl of PBS were inoculated into the left heart ventricle (day 0) of 5-week-old male nude mice (NU/NU) as previously described. Mice were separated to two groups (9 mice/group) one day before inoculating with cancer cells (day-1), then given a daily dose of 10 mg/kg of ONO-AE3-208 intraperitoneally to the treatment group and distilled water to the control group. Assessment of subsequent metastasis was monitored by measuring photon flux using the IVIS 100 in vivo imaging system 7 min after injecting luciferin intraperitoneally every 5–10 days for up to 60 days on mice anesthetized by exposure to 1–3 % isoflurane. Thirty-four 6-week old nude mice were divided into an experimental and a control group of equal number to be treated by intraperitoneal injection of ONO-AE3-208 and double distilled water, respectively. Then PC3/LUC cells were constructed by stably transfecting luciferin to prostate cancer PC3 cells and inoculated into the left ventricle of the mice to establish an animal model of systemic bone metastasis. The time of metastasis formation, photon tumor burdens, and changes of the survival curves after modeling were compared between the two groups of mice.[4] Animal model 3: Streptozotocin (STZ)-diabetic eNOS−/− mice[5] Male C57BL/6 and eNOS−/− (C57BL/6 genetic background) mice were studied at eight weeks of age. Mice received a daily i.p. injection of STZ (55 mg/kg in 0.1 M citrate buffer, pH 4.5) or citrate buffer alone after a 4 hour fast for five consecutive days. Animals received ONO-AE3-208 at a dose of 10 mg/kg/day in drinking water or drinking water alone, for three weeks beginning on the day of the first injection of STZ. In a previous report, ONO-AE3-208 administered orally to mice as a 10 mg/kg bolus achieved a peak plasma concentration of 677 ng/ml after 0.25 hours with 18% bioavailability. Urine nephrin content (Exocell, Philadelphia, PA) and urine albumin excretion were determined by ELISA after housing mice in individual metabolic cages for 24 hours. Blood glucose was determined by OneTouch UltraMini. To determine the effect of broadspectrum COX inhibition, male control and STZ-diabetic/6 and eNOS−/− mice were treated with either indomethacin (4 mg/kg/day in drinking water44, Cayman Chemical, Ann Arbor, MI) or drinking water alone beginning with the first i.p. injection of STZ and continued for two weeks (n = 10/group). Animal model 4: db/db mice[5] Male db/m and db/db mice on a BKS background aged eight weeks were randomly allocated to receive either ONO-AE3-208 (10 mg/kg/day in drinking water) or drinking water alone for eight weeks. An additional group of db/db mice were treated contemporaneously with captopril at a dose of 20 mg/kg/day in drinking water18. Blood glucose and urine albumin excretion were determined as already described. SBP was determined using a CODA non-invasive blood pressure system. Serum creatinine was determined by HPLC. For silver staining, urine volumes containing 0.5 µg creatinine were solubilized in sample buffer and separated by SDS-PAGE before staining with a ProteoSilver Stain kit. Animal model 5: Subtotally nephrectomized rats[5] Male Sprague Dawley rats aged eight weeks underwent sham or subtotal nephrectomy surgery as previously described45. Briefly, for subtotal nephrectomy surgeries, under isoflurane anesthesia, the right kidney was removed via subcapsular nephrectomy and infarction of two thirds of the left kidney was achieved by selective ligation of two out of three of the branches of the left renal artery. Sham surgery involved laparotomy and manipulation of both kidneys prior to wound closure. One week later, rats were randomized to receive ONO-AE3-208 (1 mg/kg/day or 10 mg/kg/day) in drinking water or drinking water alone and they were followed for a further seven weeks. SBP was determined by tail cuff plethysmography as previously described46. GFR was determined by single shot FITC inulin clearance with repeated sampling via the tail vein as previously described. Urine protein excretion was determined using the benzethonium chloride method after 24 hour metabolic caging and urine creatinine was determined by autoanalyzer |
| 参考文献 |
|
| 其他信息 |
Researchers used mice deficient in each of the eight types and subtypes of prostanoid receptors and examined the roles of prostanoids in dextran sodium sulfate-induced (DSS-induced) colitis. Among the prostanoid receptor-deficient mice, only EP4-deficient mice and not mice deficient in either DP, EP1, EP2, EP3, FP, IP, or TP developed severe colitis with 3% DSS treatment, which induced only marginal colitis in wild-type mice. This phenotype was mimicked in wild-type mice by administration of an EP4-selective antagonist (AE3-208). The EP4 deficiency impaired mucosal barrier function and induced epithelial loss, crypt damage, and aggregation of neutrophils and lymphocytes in the colon. Conversely, administration of an EP4-selective agonist (AE1-734) to wild-type mice ameliorated severe colitis normally induced with 7% DSS, while that of AE3-208 suppressed recovery from colitis and induced significant proliferation of CD4+ T cells. In vitro AE3-208 enhanced and AE1-734 suppressed the proliferation and Th1 cytokine production of lamina propria mononuclear cells from the colon. DNA microarray analysis revealed elevated expression of genes associated with immune response and reduced expression of genes with mucosal repair and remodeling in the colon of EP4-deficient mice. We conclude that EP4 maintains intestinal homeostasis by keeping mucosal integrity and downregulating immune response.[1]
Connecting tubule glomerular feedback (CTGF) is a mechanism in which Na reabsorption in the connecting tubule (CNT) causes afferent arteriole (Af-Art) dilation. CTGF is mediated by eicosanoids, including prostaglandins and epoxyeicosatrienoic acids; however, their exact nature and source remain unknown. We hypothesized that during CTGF, the CNT releases prostaglandin E2, which binds its type 4 receptor (EP4) and dilates the Af-Art. Rabbit Af-Arts with the adherent CNT intact were microdissected, perfused, and preconstricted with norepinephrine. CTGF was elicited by increasing luminal NaCl in the CNT from 10 to 80 mmol/L. We induced CTGF with or without the EP4 receptor blocker ONO-AE3-208 added to the bath in the presence of the epoxyeicosatrienoic acid synthesis inhibitor MS-PPOH. ONO-AE3-208 abolished CTGF (control, 9.4 ± 0.5; MS-PPOH+ONO-AE3-208, -0.6 ± 0.2 μm; P<0.001; n=6). To confirm these results, we used a different, specific EP4 blocker, L161982 (10(-5) mol/L), that also abolished CTGF (control, 8.5 ± 0.9; MS-PPOH+L161982, 0.8 ± 0.4 μm; P<0.001; n=6). To confirm that the eicosanoids that mediate CTGF are released from the CNT rather than the Af-Art, we first disrupted the Af-Art endothelium with an antibody and complement. Endothelial disruption did not affect CTGF (7.9 ± 0.9 versus 8.6 ± 0.6 μm; P=NS; n=7). We then added arachidonic acid to the lumen of the CNT while maintaining zero NaCl in the perfusate. Arachidonic acid caused dose-dependent dilation of the attached Af-Art (from 8.6 ± 1.2 to 15.3 ± 0.7 μm; P<0.001; n=6), and this effect was blocked by ONO-AE3-208 (10(-7) mol/L). We conclude that during CTGF, the CNT releases prostaglandin E2, which acts on EP4 on the Af-Art inducing endothelium-independent dilation. [2] EP4 is one of the prostaglandin E2 receptors, which is the most common prostanoid and is associated with inflammatory disease and cancer. We previously reported that over-expression of EP4 was one of the mechanisms responsible for progression to castration-resistant prostate cancer, and an EP4 antagonist ONO-AE3-208 in vivo suppressed the castration-resistant progression regulating the activation of androgen receptor. The aim of this study was to analyze the association of EP4 with prostate cancer metastasis and the efficacy of ONO-AE3-208 for suppressing the metastasis. The expression levels of EP4 mRNA were evaluated in prostate cancer cell lines, LNCaP, and PC3. EP4 over-expressing LNCaP was established, and their cell invasiveness was compared with the control LNCaP (LNCaP/mock). The in vitro cell proliferation, invasion, and migration of these cells were examined under different concentrations of ONO-AE3-208. An in vivo bone metastatic mouse model was constructed by inoculating luciferase expressing PC3 cells into left ventricle of nude mice. Their bone metastasis was observed by bioluminescent imaging with or without ONO-AE3-208 administration. The EP4 mRNA expression levels were higher in PC3 than in LNCaP, and EP4 over-expression of LNCaP cells enhanced their cell invasiveness. The in vitro cell invasion and migration were suppressed by ONO-AE3-208 in a dose-dependent manner without affecting cell proliferation. The in vivo bone metastasis of PC3 was also suppressed by ONO-AE3-208 treatment. EP4 expression levels were correlated with prostate cancer cell invasiveness and EP4 specific antagonist ONO-AE3-208 suppressed cell invasion, migration, and bone metastasis, indicating that it is a potential novel therapeutic modality for the treatment of metastatic prostate cancer. [3] Objective: To examine the effect of ONO-AE3-208, an EP4 antagonist, on the formation of bone metastasis from prostate cancer in mice. Methods: Thirty-four 6-week old nude mice were divided into an experimental and a control group of equal number to be treated by intraperitoneal injection of ONO-AE3-208 and double distilled water, respectively. Then PC3/LUC cells were constructed by stably transfecting luciferin to prostate cancer PC3 cells and inoculated into the left ventricle of the mice to establish an animal model of systemic bone metastasis. The time of metastasis formation, photon tumor burdens, and changes of the survival curves after modeling were compared between the two groups of mice. Results: At 30 days after modeling, bioluminescence imaging analysis showed that the photon tumor burdens were significantly increased in a time-dependent manner in the control group in comparison with those in the experimental group (P < 0.01). The rate of metastasis formation was significantly higher in the former than in the latter (93.3% vs 33.3%, P < 0.001). The median time of metastasis formation was 29 d (95% CI 26.547 - 35.262) in the experimental animals as compared with 21 d (95% CI 17.213 -24.787) in the controls (P < 0.001). Conclusion: EP4 antagonist ONO-AE3-208 can inhibit the formation of bone metastasis from prostate cancer in mice.[4] Connecting tubule glomerular feedback (CTGF) is a mechanism in which Na reabsorption in the connecting tubule (CNT) causes afferent arteriole (Af-Art) dilation. CTGF is mediated by eicosanoids, including prostaglandins and epoxyeicosatrienoic acids; however, their exact nature and source remain unknown. We hypothesized that during CTGF, the CNT releases prostaglandin E2, which binds its type 4 receptor (EP4) and dilates the Af-Art. Rabbit Af-Arts with the adherent CNT intact were microdissected, perfused, and preconstricted with norepinephrine. CTGF was elicited by increasing luminal NaCl in the CNT from 10 to 80 mmol/L. We induced CTGF with or without the EP4 receptor blocker ONO-AE3-208 added to the bath in the presence of the epoxyeicosatrienoic acid synthesis inhibitor MS-PPOH. ONO-AE3-208 abolished CTGF (control, 9.4 ± 0.5; MS-PPOH+ONO-AE3-208, -0.6 ± 0.2 μm; P<0.001; n=6). To confirm these results, we used a different, specific EP4 blocker, L161982 (10(-5) mol/L), that also abolished CTGF (control, 8.5 ± 0.9; MS-PPOH+L161982, 0.8 ± 0.4 μm; P<0.001; n=6). To confirm that the eicosanoids that mediate CTGF are released from the CNT rather than the Af-Art, we first disrupted the Af-Art endothelium with an antibody and complement. Endothelial disruption did not affect CTGF (7.9 ± 0.9 versus 8.6 ± 0.6 μm; P=NS; n=7). We then added arachidonic acid to the lumen of the CNT while maintaining zero NaCl in the perfusate. Arachidonic acid caused dose-dependent dilation of the attached Af-Art (from 8.6 ± 1.2 to 15.3 ± 0.7 μm; P<0.001; n=6), and this effect was blocked by ONO-AE3-208 (10(-7) mol/L). We conclude that during CTGF, the CNT releases prostaglandin E2, which acts on EP4 on the Af-Art inducing endothelium-independent dilation.[5] |
| 分子式 |
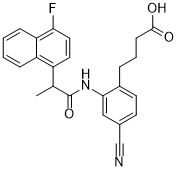
C24H21FN2O3
|
|---|---|
| 分子量 |
404.4335
|
| 精确质量 |
404.153
|
| 元素分析 |
C, 71.27; H, 5.23; F, 4.70; N, 6.93; O, 11.87
|
| CAS号 |
402473-54-5
|
| PubChem CID |
10111831
|
| 外观&性状 |
White to yellow solid powder
|
| 密度 |
1.3±0.1 g/cm3
|
| 沸点 |
662.4±55.0 °C at 760 mmHg
|
| 闪点 |
354.4±31.5 °C
|
| 蒸汽压 |
0.0±2.1 mmHg at 25°C
|
| 折射率 |
1.637
|
| LogP |
4.56
|
| tPSA |
90.19
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
5
|
| 可旋转键数目(RBC) |
7
|
| 重原子数目 |
30
|
| 分子复杂度/Complexity |
660
|
| 定义原子立体中心数目 |
0
|
| SMILES |
FC1=C([H])C([H])=C(C2=C([H])C([H])=C([H])C([H])=C21)C([H])(C([H])([H])[H])C(N([H])C1C([H])=C(C#N)C([H])=C([H])C=1C([H])([H])C([H])([H])C([H])([H])C(=O)O[H])=O
|
| InChi Key |
MTDIMKNAJUQTIO-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C24H21FN2O3/c1-15(18-11-12-21(25)20-7-3-2-6-19(18)20)24(30)27-22-13-16(14-26)9-10-17(22)5-4-8-23(28)29/h2-3,6-7,9-13,15H,4-5,8H2,1H3,(H,27,30)(H,28,29)
|
| 化学名 |
4-[4-cyano-2-[2-(4-fluoronaphthalen-1-yl)propanoylamino]phenyl]butanoic acid
|
| 别名 |
AE 3-208; AE-3-208; AE3-208; ONO AE3 208; ONO-AE3-208; 4-[4-cyano-2-[2-(4-fluoronaphthalen-1-yl)propanoylamino]phenyl]butanoic Acid; 4-Cyano-2-[[2-(4-fluoro-1-naphthalenyl)-1-oxopropyl]amino]benzenebutanoic acid; DTXSID20435810; 4-(4-Cyano-2-(2-(4-fluoronaphthalen-1-yl)propanamido)phenyl)butanoic acid; 4-Cyano-2-[[2-(4-fluoro-1-naphthalenyl)-1-oxopropyl]amino]Benzenebutanoic acid; ONO-AE-3-208; ONO-AE 3-208
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: ~33.33 mg/mL (~82.4 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (5.14 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (5.14 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL 澄清 DMSO 储备液添加到 900 μL 玉米油中并混合均匀。 View More
配方 3 中的溶解度: 5%DMSO + 40%PEG300 + 5%Tween 80 + 50%ddH2O: 4.05mg/ml (10.01mM) 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.4726 mL | 12.3631 mL | 24.7262 mL | |
| 5 mM | 0.4945 mL | 2.4726 mL | 4.9452 mL | |
| 10 mM | 0.2473 mL | 1.2363 mL | 2.4726 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
|
|
|
|