| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 50mg |
|
||
| Other Sizes |
|
| 体外研究 (In Vitro) |
在Hep3B细胞中,orphenibuta可以提高甲氨蝶呤(MTX)的抗癌功效[1]。 Hep3B 细胞可能会因羟保泰松(2.5–7.5 µM;48 小时)和 MTX (0.25–1.0 µM) 联合治疗而受到细胞毒性影响 [1]。 Orphenibuta 可以修复肝细胞 [1]。
|
|---|---|
| 体内研究 (In Vivo) |
将羟保泰松(70 mg/kg/周;口服;分两次剂量;持续 13 周)与 MTX(5.0 或 2.5 mg/kg/周;腹腔注射)联合使用可能具有抗癌作用 [1]。
|
| 细胞实验 |
细胞毒性测定[1]
细胞类型: Hep3B 细胞 测试浓度: 2.5 µM、5 µM、7.5 µM 孵育时间: 48小时 实验结果:MTX的细胞毒性增强。 |
| 动物实验 |
Animal/Disease Models: Wistar strain albino male rats (5-6 weeks; 150-220 g) [1]
Doses: 70 mg/kg/week (co-treatment with MTX 5.0 or 2.5 mg/kg/week) Route of Administration: Oral ; once a week; taken in two divided doses; for 13 weeks. Experimental Results: When combined with MTX, it has potential anti-cancer activity in rats. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
...OXYPHENBUTAZONE IS EXTENSIVELY BOUND TO PLASMA PROTEINS & HAS PLASMA HALF-TIME OF SEVERAL DAYS. ...ONLY SLOWLY EXCRETED IN URINE, SINCE BINDING TO PLASMA PROTEIN LIMITS...GLOMERULAR FILTRATION, &...RELATIVELY HIGH PKA, WHICH FAVORS PASSIVE REABSORPTION IN DISTAL TUBULE. Metabolism / Metabolites OXIDN OF PHENYLBUTAZONE DURING CHRONIC DOSING BY MEASURING URINARY EXCRETION OF 3 METABOLITES, OXYPHENBUTAZONE GAMMA-HYDROXYPHENBUTAZONE & P,GAMMA-HYDROXYPHENBUTAZONE DURING INFUSION OF HYDROCORTISONE SODIUM SUCCINATE IS REPORTED. Biological Half-Life IN MAN, OXYPHENBUTAZONE HAS BIOLOGICAL HALF-LIFE OF ABOUT 2 DAYS. |
| 毒性/毒理 (Toxicokinetics/TK) |
Interactions
SOME TRICYCLIC COMPD HAVE BEEN SHOWN TO DELAY OXYPHENYLBUTAZONE ABSORPTION IN RAT BY INCR GASTRIC EMPTYING TIME. ...OXYPHENBUTAZONE MAY PROLONG PROTHROMBIN TIME IN PT RECEIVING COUMARIN ANTICOAGULANTS CONCOMITANTLY & MAY INCR HYPOGLYCEMIC EFFECT OF INSULIN & ORAL HYPOGLYCEMIC AGENTS. WHEN OXYPHENBUTAZONE & METHANDROSTENOLONE ARE ADMIN CONCURRENTLY IN HUMANS, SERUM OXYPHENBUTAZONE LEVELS MAY BE ELEVATED. PT TREATED WITH THESE CONCURRENTLY SHOULD BE MONITORED FOR SIGNS OF OXYPHENBUTAZONE-INDUCED ADVERSE EFFECTS. OXYPHENBUTAZONE ABSORPTION...IS DECR BY DESIPRAMINE. For more Interactions (Complete) data for OXYPHENBUTAZONE (6 total), please visit the HSDB record page. |
| 参考文献 |
|
| 其他信息 |
Oxyphenbutazone is a metabolite of phenylbutazone obtained by hydroxylation at position 4 of one of the phenyl rings. Commonly used (as its hydrate) to treat pain, swelling and stiffness associated with arthritis and gout, it was withdrawn from the market 1984 following association with blood dyscrasis and Stevens-Johnson syndrome. It has a role as a non-steroidal anti-inflammatory drug, a non-narcotic analgesic, an antipyretic, a gout suppressant, a drug metabolite, a xenobiotic metabolite, an antineoplastic agent and an antimicrobial agent. It is a member of pyrazolidines and a member of phenols. It is functionally related to a phenylbutazone.
Oxyphenbutazone was withdrawn from the Canadian market in March 1985 due to concerns regarding bone marrow suppression. A non-steroidal anti-inflammatory drug. Oxyphenbutazone eyedrops have been used abroad in the management of postoperative ocular inflammation, superficial eye injuries, and episcleritis. (From AMA, Drug Evaluations Annual, 1994, p2000) It had been used by mouth in rheumatic disorders such as ankylosing spondylitis, osteoarthritis, and rheumatoid arthritis but such use is no longer considered justified owing to the risk of severe hematological adverse effects. (From Martindale, The Extra Pharmacopoeia, 30th ed, p27) Mechanism of Action ...HAS PROMINENT ANTI-INFLAMMATORY EFFECTS IN ANIMALS, & COMPARABLE EFFECTS... IN PT WITH RHEUMATOID ARTHRITIS & RELATED DISORDERS. ...INHIBITS BIOSYNTHESIS OF PROSTAGLANDINS, UNCOUPLES OXIDATIVE PHOSPHORYLATION, & INHIBITS ATP-DEPENDENT BIOSYNTHESIS OF MUCOPOLYSACCHARIDE SULFATES IN CARTILAGE. /PHENYLBUTAZONE/ Therapeutic Uses Anti-Inflammatory Agents, Non-Steroidal; Anti-Inflammatory Agents, Topical EPISCLERITIS & UVEITIS ASSOC WITH RHEUMATOID ARTHRITIS HAVE SOMETIMES BEEN TREATED SUCCESSFULLY WITH...OXYPHENBUTAZONE. ...HAS MILD URICOSURIC EFFECT IN EXPTL ANIMALS & MAN, PROBABLY ATTRIBUTABLE TO ONE OF ITS METABOLITES. ...HAS MILD URICOSURIC EFFECT IN EXPTL ANIMALS & MAN... URICOSURIC EFFECT RESULTS FROM DIMINISHED TUBULAR REABSORPTION OF URIC ACID. ...CAUSES SIGNIFICANT RETENTION OF SODIUM & CHLORIDE, ACCOMPANIED BY REDN IN URINE VOL... REDUCES UPTAKE OF IODINE BY THYROID GLAND...ALSO INHIBITS ENZYMES OF KREBS CYCLE... /PHENYLBUTAZONE/ For more Therapeutic Uses (Complete) data for OXYPHENBUTAZONE (7 total), please visit the HSDB record page. Drug Warnings ...40-YR OLD MAN RECEIVED TOTAL DOSE OF 1 G OXYPHENBUTAZONE OVER APPROX 5 DAYS, 2 MO BEFORE ADMISSION TO HOSPITAL WITH SEVERE ANEMIA & LEUCOCYTOSIS; HE DIED ON THIRD DAY AFTER DEVELOPING THIS CONDITION. ...WHITE-BLOOD CELL DISORDER CONCERNED 47-YR-OLD MAN WHO, 13 MO BEFORE ADMISSION WITH TEMP, HAD BEEN GIVEN 1.9 G OXYPHENBUTAZONE OVER 2-WK PERIOD. ON ADMISSION, HE WAS GIVEN 0.6 G OF DRUG; BLOOD EXAM SHOWED THAT HE HAD LEUKEMIA, OF WHICH HE DIED APPROX 4 MO LATER. IT SHOULD ALWAYS BE TAKEN IMMEDIATELY AFTER MEALS OR WITH FULL GLASS OF MILK, TO MINIMIZE GASTRIC IRRITATION. OXYPHENBUTAZONE IS SAID TO CAUSE SOMEWHAT LESS GASTRIC IRRITATION /THAN PHENYLBUTAZONE/. ... IT SHOULD BE TAKEN IN 3 OR 4 DIVIDED PORTIONS AFTER MEALS TO LESSEN GASTRIC IRRITATION. For more Drug Warnings (Complete) data for OXYPHENBUTAZONE (8 total), please visit the HSDB record page. |
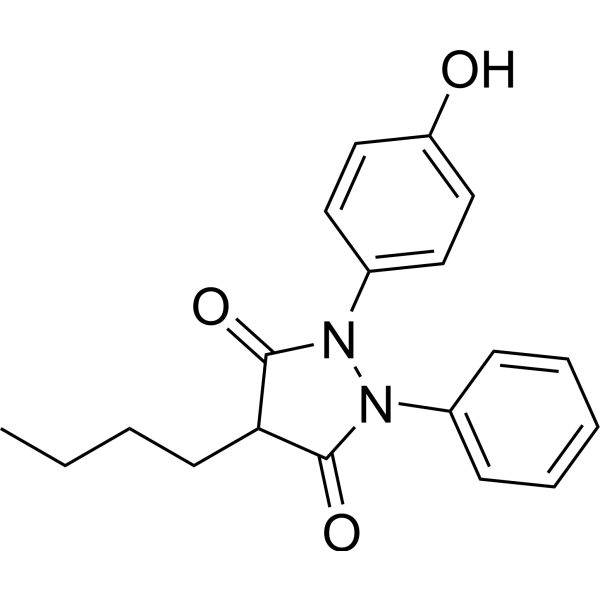
| 分子式 |
C19H20N2O3
|
|---|---|
| 分子量 |
324.38
|
| 精确质量 |
324.147
|
| CAS号 |
129-20-4
|
| 相关CAS号 |
Oxyphenbutazone monohydrate;7081-38-1;Oxyphenbutazone-d9;1189693-23-9;Oxyphenbutazone-13C6
|
| PubChem CID |
4641
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.241g/cm3
|
| 沸点 |
485.6ºC at 760mmHg
|
| 熔点 |
109-111°C
|
| 闪点 |
247.5ºC
|
| 蒸汽压 |
4.7E-10mmHg at 25°C
|
| 折射率 |
1.61
|
| LogP |
3.623
|
| tPSA |
60.85
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
3
|
| 可旋转键数目(RBC) |
5
|
| 重原子数目 |
24
|
| 分子复杂度/Complexity |
454
|
| 定义原子立体中心数目 |
0
|
| InChi Key |
HFHZKZSRXITVMK-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C19H20N2O3/c1-2-3-9-17-18(23)20(14-7-5-4-6-8-14)21(19(17)24)15-10-12-16(22)13-11-15/h4-8,10-13,17,22H,2-3,9H2,1H3
|
| 化学名 |
4-butyl-1-(4-hydroxyphenyl)-2-phenylpyrazolidine-3,5-dione
|
| 别名 |
G 29701. Oxyphenbutazone; G29701; G-29701
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~100 mg/mL (~308.29 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (7.71 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (7.71 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (7.71 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.0828 mL | 15.4140 mL | 30.8280 mL | |
| 5 mM | 0.6166 mL | 3.0828 mL | 6.1656 mL | |
| 10 mM | 0.3083 mL | 1.5414 mL | 3.0828 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。