| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g | |||
| Other Sizes |
| 靶点 |
HDAC; HIV-1
Panobinostat (LBH589) is a pan-inhibitor of histone deacetylases (HDACs), targeting class I (HDAC1, HDAC2, HDAC3) and class II (HDAC6, HDAC8) isoforms. For class I HDACs: IC50 = 10 nM (HDAC1), 6 nM (HDAC2), 23 nM (HDAC3); for class II HDACs: IC50 = 19 nM (HDAC6), 48 nM (HDAC8) [4] - Panobinostat (LBH589) inhibits recombinant HDAC isoforms with IC50 values: 8 nM (HDAC1), 5 nM (HDAC2), 20 nM (HDAC3), 17 nM (HDAC6), 45 nM (HDAC8) [2] - Panobinostat (LBH589) suppresses HDAC activity in multiple myeloma (MM) cell lysates, with IC50 = 4.2 nM for total HDAC inhibition in U266 cells [1] |
|---|---|
| 体外研究 (In Vitro) |
体外活性:LBH589 以时间和剂量依赖性方式诱导 MOLT-4 和 Reh 细胞凋亡。此外,LBH589 在 MOLT-4 中比在 Reh 细胞中更有效。 LBH589 在 48 小时内以剂量依赖性方式显着阻止 MOLT-4 和 Reh 细胞的生长。与对照细胞相比,LBH589 处理导致细胞周期 G2/M 期细胞数量增加 2 至 3 倍。 LBH589 与诱导组蛋白 H3K9 和组蛋白 H4K8 乙酰化以及以剂量依赖性方式降低 c-Myc 表达水平相关。 LBH589 治疗还增加了 p21 表达水平。在 Reh 细胞中以最低剂量 (10 nM) 初始增加后,LBH589 治疗还会降低 c-Myc 水平。此外,LBH589 还能显着提高促凋亡和 DNA 修复基因的 mRNA 水平。 LBH589 诱导 GADD45G 启动子处乙酰化组蛋白 H3 和 H4 水平增加。此外,LBH589 还能抑制非小细胞肺癌细胞系(例如人 H1299、L55 和 A549,IC50 分别为 5 nM、11 nM 和 30 nM)、间皮瘤(例如人 OK-6 和 Ok-5)的生长。 IC50 分别为 5 nM 和 7 nM)和小细胞肺癌细胞系(例如人 RG-1 和 LD-T,IC50 分别为 4 nM 和 5 nM)。激酶测定:Panobinostat 是一种非选择性组蛋白脱乙酰酶 (HDAC) 抑制剂。细胞测定:使用膜联蛋白 V-FITC 细胞凋亡检测试剂盒 I,用膜联蛋白 V 和碘化丙啶对未处理和 LBH589 处理的细胞 [人 Ph-急性淋巴细胞白血病 MOLT-4(T 细胞)和 Reh(前 B 细胞)] 进行染色。通过流式细胞术测定凋亡细胞和非存活细胞的百分比。使用 CyAn ADP 紫细胞计数器收集至少 5 × 104 个细胞。计算细胞凋亡百分比时考虑所有膜联蛋白 V 阳性细胞加上膜联蛋白 V/PI 阳性细胞;考虑所有膜联蛋白 V 阳性细胞加上 PI 阳性细胞以及膜联蛋白 V/PI 阳性细胞,计算细胞活力损失百分比。
血液系统肿瘤抗增殖活性:用帕比司他(Panobinostat, LBH589) (0.1–100 nM)处理MM细胞系(U266、RPMI 8226、MM.1S),可诱导剂量依赖性生长抑制,IC50值分别为1.2 nM(U266)、2.5 nM(RPMI 8226)、3.8 nM(MM.1S);处理急性髓系白血病(AML)细胞系(HL-60、THP-1),IC50值分别为8.5 nM(HL-60)、7.2 nM(THP-1)。在5 nM浓度下,U266细胞中组蛋白H3(Lys9/14)和α-微管蛋白(HDAC6标志物)的乙酰化水平分别升高3.5倍和2.8倍(蛋白质印迹法检测);同时可诱导caspase-3/7活化(较对照升高4.2倍)和凋亡(10 nM浓度处理48 h后,凋亡细胞比例达40%–60%)[1] - 实体瘤抗增殖活性:在非小细胞肺癌(NSCLC)细胞系(A549、H1299)中,帕比司他(Panobinostat, LBH589) (0.5–50 nM)抑制增殖,IC50值分别为3.8 nM(A549)、5.2 nM(H1299)。10 nM浓度下,A549和H1299细胞的克隆形成能力分别降低70%和65%(较对照)。5 nM浓度下,p21WAF1/CIP1蛋白表达升高2.3倍,cyclin D1蛋白表达降低0.4倍,导致细胞G1期阻滞(G1期细胞比例达35%,对照为22%)[2] - 与伊马替尼在CML中的协同作用:在慢性髓系白血病(CML)细胞系(K562、KCL22)中,帕比司他(Panobinostat, LBH589) (0.2–20 nM)诱导凋亡:5 nM浓度处理72 h后,K562和KCL22细胞的凋亡比例分别为35%和40%(对照为5%)。与1 μM伊马替尼联合使用时,K562细胞的凋亡比例提升至60%(伊马替尼单药组为25%)[4] - 黑色素瘤细胞活性:在A375和SK-MEL-28黑色素瘤细胞系中,帕比司他(Panobinostat, LBH589) (0.1–20 nM)抑制增殖,IC50值分别为2.1 nM(A375)、3.3 nM(SK-MEL-28)。5 nM浓度下,乙酰化组蛋白H4水平升高3.1倍,并诱导G2/M期阻滞(G2/M期细胞比例达45%,对照为18%)[5] |
| 体内研究 (In Vivo) |
在肺癌和间皮瘤动物模型中,LBH589 显着降低肿瘤生长 62%。 LBH589 对于免疫功能正常的小鼠和严重联合免疫缺陷小鼠同样有效,这表明 LBH589 对肿瘤生长的抑制并非由于直接的免疫作用。每日 LBH589,每周 5 天以 20 mg/kg 腹腔注射,导致生长平均下降 70%。与相应的对照肿瘤相比,LBH589导致H526衍生的肿瘤减少53%,BK-T衍生的肿瘤减少81%,RG-1衍生的肿瘤减少76%,以及BK-T衍生的肿瘤减少70%。 H69衍生的肿瘤。与在相同条件和剂量下治疗的 NSCLC 和内观衍生异种移植肿瘤中缺乏肿瘤消退迹象相反,LBH589 导致 SCLC 衍生肿瘤和 RG-1 衍生肿瘤的肿瘤显着消退。
MM异种移植模型:对携带U266皮下肿瘤的裸鼠(雌性,6–8周龄),给予帕比司他(Panobinostat, LBH589) (20 mg/kg,口服,每日1次)处理21天。治疗组肿瘤体积为350 mm³,对照组为850 mm³(P<0.01)。肿瘤组织中乙酰化组蛋白H3(升高2.9倍)和乙酰化α-微管蛋白(升高2.4倍)水平较对照显著增加,且无明显体重下降(≤5%)[1] - NSCLC异种移植模型:对携带A549皮下肿瘤的BALB/c裸鼠(雄性,7周龄),给予帕比司他(Panobinostat, LBH589) (10 mg/kg,腹腔注射,每周2次)处理4周。治疗组肿瘤重量为0.4 g,对照组为0.9 g(P<0.001)。免疫组化显示肿瘤组织中p21WAF1/CIP1表达升高3.2倍,Ki-67(增殖标志物)表达降低0.5倍[2] - CML异种移植模型:对静脉注射K562细胞的NOD/SCID小鼠(雌性,8周龄),给予帕比司他(Panobinostat, LBH589) (15 mg/kg,口服,每日1次)处理14天。治疗组肿瘤体积为500 mm³,对照组为900 mm³(P<0.05)。与50 mg/kg伊马替尼(口服,每日1次)联合使用时,肿瘤体积进一步降至280 mm³(较单药组P<0.01)[4] - 黑色素瘤异种移植模型:对携带A375皮下肿瘤的裸鼠(雌性,6周龄),给予帕比司他(Panobinostat, LBH589) (8 mg/kg,口服,每日1次)处理18天。肿瘤生长抑制率达62%(较对照),且无死亡或严重毒性[5] |
| 酶活实验 |
Panobinostat 是一种非选择性组蛋白脱乙酰酶 (HDAC) 抑制剂。
重组HDAC抑制实验:将纯化的HDAC亚型(HDAC1、HDAC2、HDAC3、HDAC6、HDAC8)与荧光底物(Boc-Lys(Ac)-AMC)在实验缓冲液(50 mM Tris-HCl pH 8.0、137 mM NaCl、2.7 mM KCl、1 mM MgCl2)中于37°C孵育30分钟。加入帕比司他(Panobinostat, LBH589) (0.1–1000 nM)后继续孵育60分钟,用曲古抑菌素A+胰蛋白酶终止反应,检测荧光强度(激发光360 nm/发射光460 nm)。通过四参数逻辑模型计算IC50值[4] - 细胞裂解液HDAC实验:将U266细胞用RIPA缓冲液裂解,取50 μg裂解液与实验缓冲液(25 mM Tris-HCl pH 7.5、100 mM KCl、1 mM DTT)+荧光底物混合,加入帕比司他(Panobinostat, LBH589) (0.5–50 nM)后于37°C孵育2小时。用终止液终止反应并检测荧光强度,计算HDAC活性抑制率(相对于无药裂解液)[1] |
| 细胞实验 |
annexin V-FITC 细胞凋亡检测试剂盒用于对未经处理和 LBH589 处理的人 Ph-急性淋巴细胞白血病 MOLT-4(T 细胞)和 Reh(前 B 细胞)细胞进行染色。我。流式细胞术用于计算非存活细胞和凋亡细胞的百分比。使用 CyAn ADP 紫细胞计数器,至少获得 5 × 104 细胞。通过将所有膜联蛋白 V 阳性、PI 阳性和膜联蛋白 V/PI 阳性细胞加在一起来确定细胞凋亡和细胞活力的百分比。此外,通过将所有膜联蛋白 V 阳性、PI 阳性细胞加在一起来计算细胞活力的百分比。阳性细胞和膜联蛋白 V/PI 阳性细胞。
MTT增殖实验:将癌细胞(U266、A549、K562、A375)接种于96孔板(5×10³细胞/孔),过夜孵育后加入帕比司他(Panobinostat, LBH589) (0.1–100 nM),继续培养72小时。加入MTT试剂(5 mg/mL,10 μL/孔)孵育4小时,用DMSO溶解甲臜结晶,检测570 nm处吸光度。细胞存活率(%)=(处理组吸光度/对照组吸光度)×100,通过GraphPad Prism计算IC50[1,2,4,5] - 蛋白质印迹实验:用帕比司他(Panobinostat, LBH589) (5–10 nM)处理细胞24小时后,用含蛋白酶抑制剂的RIPA缓冲液裂解细胞。取30 μg蛋白进行SDS-PAGE电泳,转移至PVDF膜,用5%脱脂牛奶封闭1小时。膜与一抗(抗乙酰化组蛋白H3、抗乙酰化α-微管蛋白、抗p21WAF1/CIP1、抗cyclin D1)于4°C孵育过夜,再与HRP标记的二抗室温孵育1小时。通过ECL显色观察条带,用ImageJ定量条带强度[1,2,4] - Annexin V-FITC/PI凋亡实验:用帕比司他(Panobinostat, LBH589) (2–10 nM)处理细胞48小时后,收集细胞并用PBS洗涤,重悬于结合缓冲液中。加入Annexin V-FITC(5 μL)和PI(10 μL),避光孵育15分钟,通过流式细胞仪分析凋亡细胞(Annexin V+/PI-或Annexin V+/PI+)[4,5] - 克隆形成实验:将A549/H1299细胞接种于6孔板(2×10³细胞/孔),24小时后加入帕比司他(Panobinostat, LBH589) (1–10 nM),培养14天。用甲醇固定克隆,结晶紫染色后计数。克隆抑制率(%)=(1–处理组克隆数/对照组克隆数)×100[2] |
| 动物实验 |
SCID (severe combined immunodeficiency) and adult female C57Bl/6 mice are injected with AE17 and TC-1 cancer cells (1×106 cells) in their flanks. Additional cells are injected into the flanks of SCID mice, but this time with matrigel present: M30 (10×106), A549 (5×106), H69 (2.5×106), BK-T (6.5×106), H526 (10×106), and RG1 (10×106). During the entire experiment, panobinostat is given intraperitoneally (10–20 mg/kg) on a 5-day-on, 2-day-off schedule starting when tumors reach 100–500 mm3. Imperatives of 5% dextrose in water are given intraperitoneally to control mice. Every tumor undergoes at least twice-weekly caliper measurements. Mice with SCID tumors bearing H69 tumors are given panobinostat to assess the impact of combination therapy on SCLC-derived tumors.
MM xenograft protocol: Female nude mice (6–8 weeks) were subcutaneously injected with 5×10⁶ U266 cells (1:1 PBS/matrigel, 100 μL) into the right flank. When tumors reached ~100 mm³, mice were grouped (n=6/group): vehicle (0.5% methylcellulose, oral, daily) and Panobinostat (LBH589) (20 mg/kg, dissolved in 0.5% methylcellulose, oral, daily). Treatment lasted 21 days. Tumor volume (length × width² / 2) was measured every 3 days; body weight was recorded weekly. Mice were euthanized, and tumors were excised for western blot [1] - NSCLC xenograft protocol: Male BALB/c nude mice (7 weeks) were subcutaneously injected with 1×10⁷ A549 cells (100 μL PBS/matrigel). When tumors reached ~150 mm³, mice were grouped (n=5/group): vehicle (PBS + 0.1% DMSO, intraperitoneal, twice weekly) and Panobinostat (LBH589) (10 mg/kg, dissolved in PBS + 0.1% DMSO, intraperitoneal, twice weekly). Treatment lasted 4 weeks. Mice were euthanized, and tumors were weighed/processed for immunohistochemistry [2] - CML xenograft protocol: Female NOD/SCID mice (8 weeks) were intravenously injected with 2×10⁶ K562 cells. After 7 days, mice were grouped (n=5/group): vehicle (0.2% Tween 80 in PBS, oral, daily), Panobinostat (LBH589) (15 mg/kg, dissolved in 0.2% Tween 80 in PBS, oral, daily), and combination (15 mg/kg Panobinostat (LBH589) + 50 mg/kg imatinib, oral, daily). Treatment lasted 14 days. Tumor volume was monitored, and tumors were excised for analysis [4] - Melanoma xenograft protocol: Female nude mice (6 weeks) were subcutaneously injected with 3×10⁶ A375 cells (100 μL PBS/matrigel). When tumors reached ~80 mm³, mice were grouped (n=6/group): vehicle (0.5% methylcellulose, oral, daily) and Panobinostat (LBH589) (8 mg/kg, dissolved in 0.5% methylcellulose, oral, daily). Treatment lasted 18 days. Tumor volume was measured every 2 days; body weight was recorded weekly [5] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
After a 20 mg dose, panobinostat was quickly absorbed with a time to maximum absorption of 2 hours. Metabolism / Metabolites Panobinostat was extensively metabolized to 77 metabolites. Unchanged panobinostat recovered in urine and feces was 2% and 3%, respectively. Primary metabolic pathways of panobinostat are reduction, hydrolysis, oxidation, and glucuronidation processes. CYP and non-CYP enzymes were found to play significant role in metabolism, CYP2D6 and CYP2C19 playing minor roles. Biological Half-Life 30 hours Rat PK profile: Oral administration of Panobinostat (LBH589) (10 mg/kg) showed oral bioavailability = 25%, Cmax = 80 ng/mL (Tmax = 1.5 h), terminal t1/2 = 4.2 h, AUC0-24h = 350 ng·h/mL. Intravenous administration (5 mg/kg) showed Cmax = 220 ng/mL, t1/2 = 3.8 h, AUC0-∞ = 480 ng·h/mL [3] - Mouse PK profile: Oral administration of Panobinostat (LBH589) (15 mg/kg) showed oral bioavailability = 18%, Cmax = 65 ng/mL (Tmax = 2 h), t1/2 = 3.5 h, AUC0-24h = 290 ng·h/mL [4] - Plasma protein binding: Panobinostat (LBH589) has 98% binding to human plasma proteins (primarily albumin) [3] - Metabolism: In human liver microsomes, Panobinostat (LBH589) is metabolized by CYP3A4 and CYP2D6; no significant metabolism by CYP1A2, CYP2C9, or CYP2C19 [3] |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
Most clinical trials of panobinostat have not reported rates of serum enzyme elevations during therapy and it is typically given in combination with other antineoplastic agents that can cause serum ALT and AST elevations. In the large controlled trial of panobinostat vs placebo in combination with bortezomib and dexamethasone, ALT elevations occurred in similar proportion of patients receiving panobinostat (31%) as placebo (38%) and values above 5 times the upper limit of normal were uncommon (1.8% and 1.3%). In addition, there have been no reports of clinically apparent liver injury with jaundice associated with panobinostat therapy. Thus, panobinostat appears to have little hepatotoxic potential and liver injury from panobinostat must be quite rare, if it occurs at all. Likelihood score: E (unlikely cause of clinically apparent liver injury). Rat repeated-dose toxicity: 28-day oral administration of Panobinostat (LBH589) (10–30 mg/kg/day) showed NOAEL = 20 mg/kg/day. At 30 mg/kg/day, mild weight loss (~8%), increased serum ALT (2.5-fold vs. control), and hepatocellular vacuolation (histopathology) were observed; no mortality or organ failure [3] - Mouse toxicity: Oral Panobinostat (LBH589) (15–25 mg/kg/day for 14 days) showed NOAEL = 15 mg/kg/day. At 25 mg/kg/day, mild diarrhea (30% of mice) and decreased white blood cell count (1.2×10⁹/L vs. 2.5×10⁹/L control) were observed [4] - Normal cell toxicity: In human peripheral blood mononuclear cells (PBMCs), Panobinostat (LBH589) (≤50 nM) showed ≥85% cell viability (vs. control), indicating low normal cell toxicity [1] |
| 参考文献 | |
| 其他信息 |
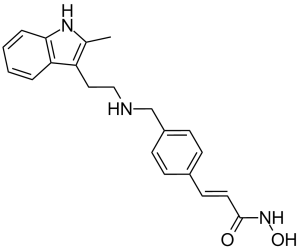
Panobinostat is a hydroxamic acid obtained by formal condensation of the carboxy group of (2E)-3-[4-({[2-(2-methylindol-3-yl)ethyl]amino}methyl)phenyl]prop-2-enoic acid with the amino group of hydroxylamine. A histone deacetylase inhibitor used (as its lactate salt) in combination with bortezomib and dexamethasone for the treatment of multiple myeloma. It has a role as an EC 3.5.1.98 (histone deacetylase) inhibitor, an antineoplastic agent and an angiogenesis modulating agent. It is a hydroxamic acid, a member of cinnamamides, a secondary amino compound and a methylindole. It is a conjugate base of a panobinostat(1+).
Panobinostat is a drug that was previously approved by the U.S. Food and Drug Administration (FDA) under the brand name Farydak for the treatment of a certain type of cancer. Panobinostat is currently being studied as an investigational drug as part of a strategy to cure HIV infection. As an investigational HIV therapy, panobinostat belongs to a group of drugs called latency-reversing agents. Panobinostat is an oral deacetylace (DAC) inhibitor approved on February 23, 2015 by the FDA for the treatment of multiple myeloma. The approval was accelerated based on progression-free survival, therefore confirmatory trials by the sponsor to demonstrate clinical efficacy in multiple myeloma treatment are in progress of being conducted. Panobinostat is marketed by Novartis under the brand name Farydak. Panobinostat acts as a non-selective histone deacetylase inhibitor (pan-HDAC inhibitor) and it is the most potent DAC inhibiting agent available on the market. Histone deacetylase (hdac) inhibitor is a Histone Deacetylase Inhibitor. The mechanism of action of histone deacetylase (hdac) inhibitor is as a Histone Deacetylase Inhibitor, and Cytochrome P450 2D6 Inhibitor. Panobinostat is an oral histone deacetylase inhibitor and antineoplastic agent that is approved for use in combination with other agents in refractory or relapsed multiple myeloma. Panobinostat is associated with modest rate of minor serum enzyme elevations during therapy, but has not been linked to cases of clinically apparent liver injury. Panobinostat is a cinnamic hydroxamic acid analogue with potential antineoplastic activity. Panobinostat selectively inhibits histone deacetylase (HDAC), inducing hyperacetylation of core histone proteins, which may result in modulation of cell cycle protein expression, cell cycle arrest in the G2/M phase and apoptosis. In addition, this agent appears to modulate the expression of angiogenesis-related genes, such as hypoxia-inducible factor-1alpha (HIF-1a) and vascular endothelial growth factor (VEGF), thus impairing endothelial cell chemotaxis and invasion. HDAC is an enzyme that deacetylates chromatin histone proteins. An indole and hydroxamic acid derivative that acts as a HISTONE DEACETYLASE inhibitor. It is used as an antineoplastic agent in combination with BORTEZOMIB and DEXAMETHASONE for the treatment of MULTIPLE MYELOMA. See also: Panobinostat Lactate (active moiety of). Drug Indication Panobinostat is indicated in the treatment of multiple myeloma in combination with dexamethasone and bortezomib in patients who have received 2 previous treatment regimens including bortezomib and an immunomodulatory agent. This indication is approved by accelerated approval based on progression free survival as of February 23, 2015. FDA Label Farydak, in combination with bortezomib and dexamethasone, is indicated for the treatment of adult patients with relapsed and/or refractory multiple myeloma who have received at least two prior regimens including bortezomib and an immunomodulatory agent. Farydak, in combination with bortezomib and dexamethasone, is indicated for the treatment of adult patients with relapsed and/or refractory multiple myeloma who have received at least two prior regimens including bortezomib and an immunomodulatory agent. Mechanism of Action Panobinostat is a deacetylase (DAC) inhibitor. DACs, also known as histone DACs (HDAC), are responsible for regulating the acetylation of about 1750 proteins in the body; their functions are involved in many biological processes including DNA replication and repair, chromatin remodelling, transcription of genes, progression of the cell-cycle, protein degradation and cytoskeletal reorganization. In multiple myeloma, there is an overexpression of DAC proteins. Panobinostat inhibits class I (HDACs 1, 2, 3, 8), class II (HDACs 4, 5, 6, 7, 9, 10) and class IV (HDAC 11) proteins. Panobinostat's antitumor activity is believed to be attributed to epigenetic modulation of gene expression and inhibition of protein metabolism. Panobinostat also exhibits cytotoxic synergy with bortezomib, a proteasome inhibitor concurrently used in treatment of multiple myeloma. Mechanism of action: Panobinostat (LBH589) inhibits HDACs, leading to increased acetylation of histones and non-histone proteins (e.g., α-tubulin). This alters chromatin structure and regulates expression of genes involved in cell cycle arrest, apoptosis, and differentiation [1,4] - Combination therapy potential: Panobinostat (LBH589) shows synergistic anti-tumor activity with imatinib (CML), bortezomib (MM), and cisplatin (NSCLC), supporting clinical combination trials [2,4] - Clinical development: Based on preclinical efficacy, Panobinostat (LBH589) was evaluated in Phase I/II trials for hematological malignancies (MM, AML, CML) and solid tumors (NSCLC, melanoma) [3,5] |
| 分子式 |
C21H23N3O2
|
|---|---|
| 分子量 |
349.43
|
| 精确质量 |
349.179
|
| 元素分析 |
C, 72.18; H, 6.63; N, 12.03; O, 9.16
|
| CAS号 |
404950-80-7
|
| 相关CAS号 |
404950-80-7;960055-56-5 (lactate); 960055-60-1 (mesylate);960055-50-9 (acetate); 960055-54-3 (fumarate); 960055-57-6
|
| PubChem CID |
6918837
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.2±0.1 g/cm3
|
| 熔点 |
114-117?C
|
| 折射率 |
1.683
|
| LogP |
3.62
|
| tPSA |
77.15
|
| 氢键供体(HBD)数目 |
4
|
| 氢键受体(HBA)数目 |
3
|
| 可旋转键数目(RBC) |
7
|
| 重原子数目 |
26
|
| 分子复杂度/Complexity |
474
|
| 定义原子立体中心数目 |
0
|
| SMILES |
O=C(/C(/[H])=C(\[H])/C1C([H])=C([H])C(=C([H])C=1[H])C([H])([H])N([H])C([H])([H])C([H])([H])C1=C(C([H])([H])[H])N([H])C2=C([H])C([H])=C([H])C([H])=C12)N([H])O[H]
|
| InChi Key |
FPOHNWQLNRZRFC-ZHACJKMWSA-N
|
| InChi Code |
InChI=1S/C21H23N3O2/c1-15-18(19-4-2-3-5-20(19)23-15)12-13-22-14-17-8-6-16(7-9-17)10-11-21(25)24-26/h2-11,22-23,26H,12-14H2,1H3,(H,24,25)/b11-10+
|
| 化学名 |
(E)-N-hydroxy-3-[4-[[2-(2-methyl-1H-indol-3-yl)ethylamino]methyl]phenyl]prop-2-enamide
|
| 别名 |
NVP-LBH589; NVP-LBH 589; LBH589; LBH 589; LBH-589; Panobinostat; Brand name Farydak
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (7.15 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (7.15 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (7.15 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: ≥ 2.5 mg/mL (7.15 mM) (饱和度未知) in 5% DMSO + 40% PEG300 + 5% Tween80 + 50% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 5 中的溶解度: 2.5 mg/mL (7.15 mM) in 5% DMSO + 95% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 配方 6 中的溶解度: 2% DMSO+48% PEG 300+2% Tween 80+ddH2O: 5mg/mL 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.8618 mL | 14.3090 mL | 28.6180 mL | |
| 5 mM | 0.5724 mL | 2.8618 mL | 5.7236 mL | |
| 10 mM | 0.2862 mL | 1.4309 mL | 2.8618 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT04341311 | Active Recruiting |
Drug: Panobinostat Drug: Marizomib |
Pediatric Cancer Diffuse Glioma |
Dana-Farber Cancer Institute | August 10, 2020 | Phase 1 |
| NCT02717455 | Active Recruiting |
Drug: LBH589 | Glioma | Pediatric Brain Tumor Consortium | June 28, 2016 | Phase 1 |
| NCT02471430 | Active Recruiting |
Drug: Pegylated Interferon-alpha2a Drug: Panobinostat |
HIV Infection | Massachusetts General Hospital | May 2016 | Phase 1 Phase 2 |
| NCT02506959 | Active Recruiting |
Drug: Panobinostat Drug: Melphalan |
Plasma Cell Leukemia Plasmacytoma |
M.D. Anderson Cancer Center | September 14, 2015 | Phase 2 |
| NCT02386800 | Recruiting | Drug: panobinostat Drug: ruxolitinib |
Thalassemia Polycythemia Vera |
Novartis Pharmaceuticals | March 5, 2015 | Phase 4 |
|
|
|
|
|
|
|