| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 5g |
|
||
| 10g |
|
||
| Other Sizes |
|
| 靶点 |
PARP ( IC50 = 50 nM )
|
|
|---|---|---|
| 体外研究 (In Vitro) |
体外活性:3-Aminobenzamide (>1 μM) 可抑制 95% 以上的 PARP 活性,且没有明显的细胞毒性。 INO-1001 通过阻断辐射部分之间发生的大部分 DNA 修复来显着敏化 CHO 细胞。暴露于 400 μM H2O2 后,3-Aminobenzamide 可通过增强乙酰胆碱诱导的、内皮依赖性的、一氧化氮介导的血管舒张作用来显着改善内皮功能。 激酶测定:使用 PARP 活性测定试剂盒测量 PARP 活性。该方法通过在存在剪切的基因组 DNA 的情况下确定 3H-NAD 掺入三氯乙酸 (TCA) 可沉淀材料(激活 PARP)的水平来测量相对 PARP 活性。将反应混合物直接添加到 12 孔培养板中经洗涤的培养物中,并让反应在 37°C 下进行 60 分钟,然后机械取出细胞,转移到微量离心管中,并用冰冷的 5% 沉淀三氯乙酸。细胞测定:3-Aminobenzamide (PARP-IN-1) 是一种有效的 PARP 抑制剂,在 CHO 细胞中的 IC50 约为 50 nM,并且在再灌注过程中充当氧化剂诱导的肌细胞功能障碍的介质。
|
|
| 体内研究 (In Vivo) |
在 db/db (Leprdb/db) 小鼠模型中,3-Aminobenzamide 可改善糖尿病引起的白蛋白排泄和系膜扩张,并减少糖尿病引起的足细胞耗竭[3]。 3-氨基苯甲酰胺(通过脑内注射 1.6 毫克/千克)可防止 NAD+ 消耗并改善小鼠受控皮质冲击 (CCI) 后的水迷宫性能
|
|
| 酶活实验 |
使用 PARP 活性检测试剂盒可测定 PARP 活性。在存在激活 PARP 的剪切基因组 DNA 的情况下,使用此方法测量掺入三氯乙酸 (TCA) 可沉淀材料中的 3H-NAD 量,以确定相对 PARP 活性。使反应在 37°C 下进行 60 分钟后,将反应混合物直接添加到 12 孔培养板中经洗涤的培养物中。然后机械取出细胞,移至微量离心管中,并用冰冷的 5% TCA 沉淀。
|
|
| 细胞实验 |
3-Aminobenzamide (PARP-IN-1) 是一种有效的 PARP 抑制剂,在再灌注过程中充当氧化剂诱导的肌细胞功能障碍的介质。其在 CHO 细胞中的 IC50 约为 50 nM。
|
|
| 动物实验 |
|
|
| 药代性质 (ADME/PK) |
Metabolism / Metabolites
3-Aminobenzamide (3-ABA) is a potent radiosensitizer that inhibits the repair of DNA strand breaks. The aim of this study was to monitor the biodistribution and pharmacokinetics of a fluorinated 3-ABA derivative in tumor-bearing rats by magnetic resonance imaging (MRI). To this end, 3-ABA was labeled with fluorine-19 by trifluoroethylation [3-amino-N-2,2,2-trifluoroethylbenzamide (3-ABA-TFE)], which only slightly increased the cytotoxicity of the compound as demonstrated by colony-forming assays. After intraperitoneal injection of 400 mg/kg BW 3-ABA-TFE to nine Copenhagen rats with Dunning prostate adenocarcinoma, (19)F MR images were acquired at a whole-body MR system with a spatial sampling of 10 x 10 x 15 mm(3). While 3-ABA-TFE was observed in all major organs and the muscular system, only a small and heterogeneous signal could be detected in the adenocarcinoma. Serial MR measurements yielded maximum tissue signals about 2 days after 3-ABA-TFE administration. At this time point, the mean muscle-to-liver and tumor-to-liver signal ratio was 0.31+/-0.07 and 0.11+/-0.04, respectively. Application of the (19)F MRI strategy makes it possible to measure the biodistribution and pharmacokinetics of 3-ABA-TFE in individual animals in a longitudinal manner. The results obtained for the prostate adenocarcinoma indicate that delivery of 3-ABA-TFE to solid tumors may be seriously hampered by tumor-specific factors and that the intratumoral uptake of the substance may be lower than in normal tissues. Therefore, the development of effective carrier systems is mandatory to improve tumor-selective delivery. |
|
| 毒性/毒理 (Toxicokinetics/TK) |
Interactions
Chronic irradiation (three times a week) with ultraviolet B light of the skin of hairless mouse Uscd (Hr) strains resulted in the induction of skin tumors after 25 to 41 weeks. Topical applications of 3-aminobenzamide (3AB; 0.1 or 1 M) after each irradiation significantly shortened the earliest time of onset of tumors to 13 to 25 weeks and increased the number of animals that developed tumors over 41 weeks from 67% without 3AB to 73% and 81% with 0.1 and 1 M 3AB, respectively. ... The poly(ADP-ribosyl)transferase inhibitor, 3-aminobenzamide (3-ABA), reduced morphological evidence of 1,2-dibromo-3-chloropropane (DBCP)-induced DNA damage determined by alkaline elution. The DBCP plasma, kidney, and testis tissue doses determined between 1 and 8 hr after a single intraperitoneal injection were somewhat higher with than without 3-ABA pretreatment. Furthermore, the amount of DBCP metabolites covalently bound to macromolecules was reduced to about 20-30 percent of control, indicating that 3-ABA may have an effect on the formation/detoxication of reactive DBCP metabolites. ... ... 3-aminobenzamide (3AB) combined with X-rays was used to evaluate the micronucleus dose-response relationship in /in vitro-irradiated lymphocytes/ blood from 14 individuals. While it is known that 3AB inhibits poly(ADP-ribose) polymerase activity in vitro, ... it also increases the X-ray-induced micronucleus yields. The resulting dose-response relationship varies from subject to subject. ... Gene expression of human immunodeficiency virus type 1 (HIV-1) is induced not only by trans activation mediated through a gene product (tat) encoded by the virus but also by treatment of virus-carrying cells with DNA-damaging agents such as UV light. Employing an artificially constructed DNA in which the chloramphenicol acetyltransferase gene was placed under the control of the HIV-1 long terminal repeat, ... the induction process in HeLa cells /was analyzed/ and ... inhibitors of poly(ADP-ribose) polymerase /including 3-aminobenzamide/ suppressed UV-induced HIV-1 gene expression but not tat-mediated expression. ... Suppression occurs at the posttranscriptional level. These results indicate that HIV-1 gene expression is activated by at least two different mechanisms, one of which involves poly-ADP ribosylation. ... Synergistically enhanced sister chromatid exchange (SCE) frequency by cyclophosphamide (CP) was observed when L1210 lymphoid tumor cells were exposed in vivo to a non-toxic concentration of 3-aminobenzamide (3-AB). Additive effects in SCE induction in vivo were observed when either Ehrlich ascites tumor (EAT) cells or P388 lymphocytic leukemia cells treated with CP were exposed to 3-AB in vivo. 3-AB enhanced the survival time of L1210 tumor bearing BDF1 mice treated with CP. However, the combined CP plus 3-AB treatment did not increase the survival of either EAT BALB/c- or P388 BDF1-tumor bearing mice compared with the effect on survival by CP alone. Therefore the in vivo differential antitumor effect, by CP in conjunction with 3-AB, appears to correlate well with the in vivo differential effect on cytogenetic damage caused by the combined CP plus 3-AB treatment. In the Salmonella typhimurium/mammalian microsome test CP appears to have a dose dependent ability to induce base-pair substitutions in strains TA 100 and TA 1535 and frameshift mutations in strains TA 98 and TA 1537. Both types of mutation were synergistically increased in the presence of 3-AB. |
|
| 参考文献 |
|
|
| 其他信息 |
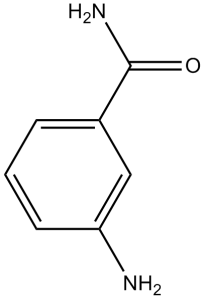
3-aminobenzamide is a substituted aniline that is benzamide in which one of the meta- hydrogens is replaced by an amino group. It has a role as an EC 2.4.2.30 (NAD(+) ADP-ribosyltransferase) inhibitor. It is a member of benzamides and a substituted aniline.
Mechanism of Action Poly(ADP-ribosyl)transferase inhibitor A 3 hr exposure to 1 mM H2O2 followed by 6 hr post-challenge growth in peroxide-free medium induces necrosis in U937 cells. Addition of the poly(ADP-ribose)polymerase inhibitor 3-aminobenzamide during recovery prevents necrosis and triggers apoptosis, as shown by the appearance of apoptotic bodies, extensive blebbing and formation of multimeric DNA fragments as well as 50 kb double stranded DNA fragments. Thus, the same initial damage can be a triggering event for both apoptotic and necrotic cell death. Furthermore, necrosis does not appear to be a passive response to overwhelming damage. Treatment with 3-aminobenzamide, known as an inhibitor of poly(ADP-ribose)polymerease, decreased the toxicity and covalent binding of the herbicide dichlobenil (2,6-dichlorobenzonitrile; 12 mg/kg; i.p.) in the mouse olfactory mucosa. In vitro studies showed that 3-aminobenzamide markedly reduced the NADPH-dependent covalent binding of [14C]dichlobenil and the hydroxylation of p-nitrophenol which have previously been suggested to be mediated by a common form of cytochrome P450 (P450) in rat olfactory microsomes ... . Furthermore, 3-aminobenzamide markedly reduced the P450-dependent metabolic activation of [3H]NNK (4-(N-methyl-N-nitrosamino)-1-(3-pyridyl)-1-butanone) in rat olfactory microsomes and slightly decreased the P450 2B1-dependent pentoxyresorufindealkylase activity in liver microsomes of phenobarbital-treated rats. The present results suggest that 3-aminobenzamide is also an inhibitor of P450 and that the lack of toxicity of dichlobenil in the olfactory mucosa of 3-aminobenzamide-treated mice is related to a decreased metabolic activation of dichlobenil at this site... . ... Cll lines deficient in poly(ADP-ribose) synthesis due to deficiency in the enzyme poly(ADP-ribose) polymerase (PADPRP) or depletion of its substrate NAD+ overexpress GRP78. Furthermore, this overexpression of GRP78 is associated with the acquisition of resistance to topoisomerase II-directed drugs such as etoposide (VP-16) ... Thus, /the/ studies suggest that interference with NAD+-PADPRP metabolism could provide an important approach to (a) define pathways of GRP78 induction, (b) study the effect of GRP78 on other cellular processes, (c) elucidate the mechanism of GRP78-dependent resistance to topoisomerase II targeted drugs, and (d) modulate responses to chemotherapy in normal and tumor tissues. However, in the in vivo situation, it is impractical to interfere with NAD+-PADPRP metabolism by mutational inactivation of PADPRP or by depletion of its substrate NAD+. Therefore, ... several inhibitors of NAD+-PADPRP metabolism including 3-aminobenzamide, PD128763, and 6-aminonicotinamide /were examined/ for their ability to reproduce the results obtained with cell lines deficient in NAD+-PADPRP metabolism relative to the induction of GRP78 and subsequent development of resistance to VP-16. ... 6-aminoicotinamide treatment is highly effective in the induction of GRP78 and subsequent development of resistance to VP-16, whereas treatment with 3-aminobenzamide or PD128763 does not induce GRP78 and thus does not result in VP-16 resistance. For more Mechanism of Action (Complete) data for 3-AMINOBENZAMIDE (7 total), please visit the HSDB record page. Therapeutic Uses /EXPL THER/: Poly (ADP-ribose) polymerase (PARP), a nuclear enzyme activated by strand breaks in DNA, plays an important role in the colon injury associated with experimental colitis. The aim of the present study was to examine the effects of 3-aminobenzamide (3-AB), an inhibitor of PARP activity, in the development of acute pancreatitis caused by cerulein in mice. Intraperitoneal injection of cerulein in mice resulted in severe, acute pancreatitis characterized by edema, neutrophil infiltration and necrosis and elevated serum levels of amylase and lipase. Infiltration of pancreatic and lung tissue with neutrophils (measured as increase in myeloperoxidase activity) was associated with enhanced expression of the intercellular adhesion molecule-1 (ICAM-1) and P-selectin. Immunohistochemical examination demonstrated a marked increase in the staining (immunoreactivity) for transforming growth factor-beta (TGF-beta) and vascular endothelial growth factor (VEGF) in the pancreas of cerulein-treated mice in comparison to sham-treated mice. Acute pancreatitis in vehicle-treated mice was also associated with a significant mortality (40% survival at 5 days after cerulein administration). In contrast, (1) the degree of pancreatic inflammation and tissue injury (histological score), (2) upregulation/formation of ICAM-1 and P-selectin, (4) neutrophils infiltration and (5) the expression of TGF-beta and VEGF was markedly reduced in pancreatic tissue obtained from cerulein-treated mice which have been treated with 3-AB. These findings provide the evidence that PARP inhibition reduces the degree of pancreas injury caused by acute pancreatitis induced by cerulein administration. /EXPL THER/: Poly(ADP-ribose) polymerase (PARP) is a nuclear enzyme which plays an important role in regulating cell death and cellular responses to DNA repair. Pharmacological inhibitors of PARP are being considered as treatment for cancer both in monotherapy as well as in combination with chemotherapeutic agents and radiation, and were also reported to be protective against untoward effects exerted by certain anticancer drugs. ... pharmacological inhibition of PARP with 3-aminobenzamide or PJ-34 dose-dependently reduces VEGF-induced proliferation, migration, and tube formation of human umbilical vein endothelial cells in vitro. These results suggest that treatment with PARP inhibitors may exert additional benefits in various cancers and retinopathies by decreasing angiogenesis. /EXPL THER/: The activation of poly (ADP-ribose) polymerase (PARP) plays a pivotal role in mediating N-methyl-N-nitrosourea (MNU)-induced photoreceptor cell apoptosis. ... the retinoprotective effects of the PARP inhibitor 3-aminobenzamide (3-AB) against MNU-induced retinal damage in relation to dose and timing of prescription, and the involvement of the transcription factor nuclear factor (NF)-kappaB /were examined/. Female Sprague-Dawley rats were intraperitoneally injected with 60 mg/kg MNU at 50 days of age, and were then immediately given a subcutaneous injection of 0, 1, 5, 10, 30 or 50 mg/kg of 3-AB, or were injected with 50 mg/kg 3-AB 12h before, concurrently, or 4, 6 or 12h after MNU. Rats were killed 3 and 7 days after MNU, and MNU-treated and 3-AB-injected retinas were compared with MNU-untreated control retinas or MNU-treated/3-AB-uninjected retinas. Apoptosis in photoreceptor cells was detected by performing formamide-induced DNA denaturation and staining with anti-single-stranded DNA antibody. Retinal morphologies were compared and evaluated morphometrically using the photoreceptor cell ratio and retinal damage ratio as indices to evaluate the efficacy of 3-AB. ... expression of the phosphorylated form of NF-kappaB and IkappaBalpha (p-NF-kappaB and p-IkappaBalpha, respectively) in retinas of MNU-treated rats concurrently treated with or without 50mg/kg 3-AB, compared with MNU-untreated control retinas /was examined/. 3-AB dose-dependently suppressed photoreceptor cell apoptosis: 50mg/kg 3-AB injected concurrently with MNU completely rescued photoreceptor cell damage; 30 mg/kg 3-AB significantly reduced photoreceptor cell damage; 10 mg/kg 3-AB tended to suppress photoreceptor cell damage; /EXPL THER/: Poly(ADP-ribose) polymerases (PARPs) are defined as a family of cell signaling enzymes present in eukaryotes, which are involved in poly(ADP-ribosylation) of DNA-binding proteins. The best studied of these enzymes (PARP-1) is involved in the cellular response to DNA damage so that in the event of irreparable DNA damage overactivation of PARP-1 leads to necrotic cell death. Inhibitors of PARP-1 activity in combination with DNA-binding antitumor drugs may constitute a suitable strategy in cancer chemotherapy. When DNA is moderately damaged, PARP-1 participates in the DNA repair process and the cell survives. However, in the case of extensive DNA damage PARP-1 overactivation induces a decrease of NAD+ and ATP levels leading to cell dysfunction or even to necrotic cell death. So, due to PARP-1 involvement in cell death, pharmacological inhibition of PARP-1 activity by PARP-1 inhibitors may constitute a suitable target to enhance the activity of antitumor drugs through inhibition of necrosis and activation of apoptosis. PARP-1 inhibitors such as 3-aminobenzamide, 1,5-dihydroxyisoquinolinone and the recently patented tryciclic benzimidazoles have shown potent inhibitory effects of PARP-1 activity in tumor cells. For more Therapeutic Uses (Complete) data for 3-AMINOBENZAMIDE (7 total), please visit the HSDB record page. |
| 分子式 |
C₇H₈N₂O
|
|
|---|---|---|
| 分子量 |
136.15
|
|
| 精确质量 |
136.063
|
|
| CAS号 |
3544-24-9
|
|
| 相关CAS号 |
|
|
| PubChem CID |
1645
|
|
| 外观&性状 |
Off-white to light brown solid powder
|
|
| 密度 |
1.2±0.1 g/cm3
|
|
| 沸点 |
329.6±25.0 °C at 760 mmHg
|
|
| 熔点 |
115-116 °C(lit.)
|
|
| 闪点 |
153.2±23.2 °C
|
|
| 蒸汽压 |
0.0±0.7 mmHg at 25°C
|
|
| 折射率 |
1.633
|
|
| LogP |
0.33
|
|
| tPSA |
69.11
|
|
| 氢键供体(HBD)数目 |
2
|
|
| 氢键受体(HBA)数目 |
2
|
|
| 可旋转键数目(RBC) |
1
|
|
| 重原子数目 |
10
|
|
| 分子复杂度/Complexity |
136
|
|
| 定义原子立体中心数目 |
0
|
|
| InChi Key |
GSCPDZHWVNUUFI-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C7H8N2O/c8-6-3-1-2-5(4-6)7(9)10/h1-4H,8H2,(H2,9,10)
|
|
| 化学名 |
3-aminobenzamide
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
||
|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 25 mg/mL (183.62 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶。
配方 2 中的溶解度: 30% propylene glycol+ 5% Tween 80+ 65% D5W: 30 mg/mL (220.35mM) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 7.3448 mL | 36.7242 mL | 73.4484 mL | |
| 5 mM | 1.4690 mL | 7.3448 mL | 14.6897 mL | |
| 10 mM | 0.7345 mL | 3.6724 mL | 7.3448 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。