| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Pirimiphos-methyl is known to be absorbed through intact skin, from the GI tract, & by inhalation. Oral administration of 2-(14)C-ring-labelled pirimiphos-methyl at a dose of 0.6 mg/kg bw to five male rats resulted in a mean urinary excretion of 80.7% and mean fecal excretion of 7.3% in 24 hr, indicating rapid absorption. At 96 h, 86.0% and 15.2% of the administered dose had been excreted in urine and feces, respectively. Nine (unidentified) metabolites were present in the urine. Female rats given 2-(14)C-pirimiphos-methyl at a dose of 7.5 mg/kg bw orally were bled (cardiac puncture, three rats per time interval) at 0.5, 1, 3, 5, 7 or 24 hr after dosing. Maximum blood concentrations (at 0.5 hr) were 2-3 ug/mL, declining by 50% 1 hr after dosing. By 24 hr, concentrations of (14)C in blood were 0.2-0.3 ug/mL, and of pirimiphos-methyl, 0.01-0.02 ug/mL. Rats treated for 4 days with 2-(14)C-pirimiphos-methyl at a dose of 7.5 mg/kg bw per day and sacrificed at intervals of 24 hr did not show any increase in blood concentrations with time. Tissue concentrations of total radioactivity in the liver, kidney and fat over the 4 days were generally less than 2 mg pirimiphos-methyl equivalents/kg tissue (concentrations of unchanged pirimiphos-methyl being less than 0.15 mg/kg tissue). There was no evidence of tissue accumulation. Adult male Wistar rats were intubated with (14)C-labelled pirimiphos-methyl at a dose of 1 mg/kg bw per day. Four groups of three animals were dosed for 3, 7, 14 or 21 days and sacrificed 24 hr after the final dose. A further five groups of three rats were given similar doses for 28 days and sacrificed 1, 3, 7, 14, or 28 days after dosing. For each of the nine groups, one rat that did not receive pirimiphos-methyl was used as a control. After sacrifice, samples of liver, kidney, muscle, fat, erythrocytes and plasma were taken for analyses. Urine and feces were collected from two rats during the 24 hr after the seventh dose. Recovery of (14)C from (14)C-labelled pirimiphos-methyl added to control tissues was 96.9 +/- 5.2%. In all tissue samples taken at all time intervals, the concentration of radioactivity was very low, close to or below detection limits. Concentrations did not increase with repeated dosing. Liver concentrations were fairly constant (0.03 ppm) and similar concentrations were detected in some kidney samples. In other tissues, the concentration of radioactivity was generally below the limits of detection (0.04-0.06 ppm). Three days after cessation of dosing, one animal had detectable concentrations of radioactivity in the kidney. At 7 days and on subsequent days, no residues were found. Excretion was between 70% and 80% of a single dose, after administration of seven consecutive doses, providing evidence for rapid metabolism and elimination rather than poor absorption. For more Absorption, Distribution and Excretion (Complete) data for PIRIMIPHOS-METHYL (6 total), please visit the HSDB record page. Metabolism / Metabolites Twelve metabolites of pirimiphos-methyl were separated by thin-layer chromatography from the urine of rats & a dog. No unchanged compound was detected & no metabolite had anticholinesterase activity. Briefly, the P-O bond is cleaved extensively & N-dealkylation &/or conjugation is a further step in the metabolism of the pyrimidine leaving group. The mechanism by which large repeated doses of pirimiphos-methyl reduces the hemoglobin of rats is unknown. It may be caused by 2-diethylamino-4-hydroxy-6-methylpyrimidine, a metabolite formed by both mammals and plants. Although this metabolite has an acute toxicity of the same order of magnitude as the parent compound, it was (unlike the parent compound) tolerated by rats at a dosage of 400 mg/kg for 2 wk; even so, its action on the blood was indicated by an incr in reticulocytes & a decr in lymphocytes. Metabolism of organophosphates occurs principally by oxidation, by hydrolysis via esterases and by reaction with glutathione. Demethylation and glucuronidation may also occur. Oxidation of organophosphorus pesticides may result in moderately toxic products. In general, phosphorothioates are not directly toxic but require oxidative metabolism to the proximal toxin. The glutathione transferase reactions produce products that are, in most cases, of low toxicity. Paraoxonase (PON1) is a key enzyme in the metabolism of organophosphates. PON1 can inactivate some organophosphates through hydrolysis. PON1 hydrolyzes the active metabolites in several organophosphates insecticides as well as, nerve agents such as soman, sarin, and VX. The presence of PON1 polymorphisms causes there to be different enzyme levels and catalytic efficiency of this esterase, which in turn suggests that different individuals may be more susceptible to the toxic effect of organophosphate exposure. Biological Half-Life Female rats given 2-(14)C-pirimiphos-methyl at a dose of 7.5 mg/kg bw orally were bled (cardiac puncture, three rats per time interval) at 0.5, 1, 3, 5, 7 or 24 hr after dosing. Maximum blood concentrations (at 0.5 hr) were 2-3 ug/mL, declining by 50% 1 hr after dosing. |
|---|---|
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
Pirimiphos-methyl is a cholinesterase or acetylcholinesterase (AChE) inhibitor. A cholinesterase inhibitor (or 'anticholinesterase') suppresses the action of acetylcholinesterase. Because of its essential function, chemicals that interfere with the action of acetylcholinesterase are potent neurotoxins, causing excessive salivation and eye-watering in low doses, followed by muscle spasms and ultimately death. Nerve gases and many substances used in insecticides have been shown to act by binding a serine in the active site of acetylcholine esterase, inhibiting the enzyme completely. Acetylcholine esterase breaks down the neurotransmitter acetylcholine, which is released at nerve and muscle junctions, in order to allow the muscle or organ to relax. The result of acetylcholine esterase inhibition is that acetylcholine builds up and continues to act so that any nerve impulses are continually transmitted and muscle contractions do not stop. Among the most common acetylcholinesterase inhibitors are phosphorus-based compounds, which are designed to bind to the active site of the enzyme. The structural requirements are a phosphorus atom bearing two lipophilic groups, a leaving group (such as a halide or thiocyanate), and a terminal oxygen. Toxicity Data LC (rat) > 5040 mg/m3/4h Interactions The effect of L-ascorbic acid supplementation on Pirimiphos-methyl induced toxicity was studied in albino rats. Biochemical estimations were made in rats administered orally the insecticide at 100 and 200 mg/kg body weight with or without oral supplementation of L-ascorbic acid at 200 mg/kg b.w. The biochemical assessments included estimations of brain and plasma cholinesterases, levels of ascorbic acid in liver, kidney and adrenals, urinary levels of ascorbic acid and glucuronic acid. A lower degree of inhibition of the cholinesterases was evident in ascorbic acid supplemented rats. Marked elevation in urinary levels of ascorbic acid and glucuronic acid was observed in the insecticide treated rats. Results of this study suggests that L-ascorbic acid supplementation partially offsets Pirimiphos-methyl induced toxicity Technical hexachlorocyclohexane (100 mg/kg/d) and pirimiphosmethyl EC 50 (250 mg/kg/d) given individually and in combination to female rats for 7, 15 or 30 d by skin application caused poisoning, pathomorphological changes in vital organs, and significant enzymatic changes in liver and serum. The changes produced by the 2 compounds in combination did not suggest potentiation at the tested dose levels. The pesticides benomyl, a benzimidazole fungicide, and pirimiphos-methyl, an organophosphorus insecticide, were tested separately and in combination at a ratio of 6:1, a mixture frequently found in foodstuffs by residual analysis, to determine their possible genotoxic action. The effect was measured by the micronucleus test carried out on cultured rat hepatocytes stimulated to proliferate by epidermal growth factor (EGF). Adult rat hepatocytes were exposed in vitro for 48 hr to the substances at increasing non-cytotoxic doses, chosen on the basis of cytotoxicity tests such as LDH and Neutral red assays. Benomyl induced a significant dose-related increase in micronucleus frequency; in contrast, pirimiphos-methyl was not genotoxic at any dose tested. When the hepatocytes were exposed to the two pesticides together at increasing doses, an enhancement in micronucleus frequency similar to that of benomyl alone was found, indicating that at this ratio and non-cytotoxic doses (up to 25 micrograms/mL benomyl + 4.2 micrograms/mL pirimiphos-methyl) no interaction occurs. Non-Human Toxicity Values LD50 Rat oral 1250 mg/kg LD50 Mouse oral 1180 mg/kg LD50 Rabbit oral 1150 mg/kg LD50 Guinea pig oral 1000 mg/kg For more Non-Human Toxicity Values (Complete) data for PIRIMIPHOS-METHYL (15 total), please visit the HSDB record page. |
| 参考文献 |
|
| 其他信息 |
Pirimiphos-methyl is a yellow liquid. Corrosive to tin and mild steel. Used as an insecticide.
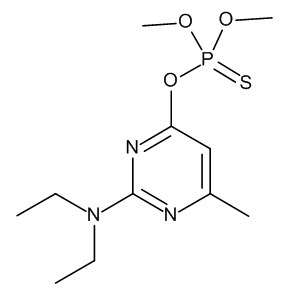
Pirimiphos-methyl is an organic thiophosphate that is O,O-dimethyl O-pyrimidin-4-yl phosphorothioate substituted by a methyl group at position 6 and a diethylamino group at position 2. It has a role as an EC 3.1.1.7 (acetylcholinesterase) inhibitor, an acaricide, an agrochemical, an insecticide and an environmental contaminant. It is an organic thiophosphate and an aminopyrimidine. It is functionally related to a 2-diethylamino-6-methylpyrimidin-4(1H)-one. Pirimiphos-methyl is an organophosphate and fumigant insecticide with pirimiphos-methyl and no longer in production. It is used to control a wide range of insects and mites in stores, animal houses, domestic and industrial premisies. It is considered to be an acetylcholinesterase (AChE) inhibitor with contact and respiratory action. This is one of several compounds used for vector control of Triatoma. Mechanism of Action Systemically, pirimiphos-methyl inhibits cholinesterase, & this is the only known mechanism of its toxic action. In spite of its rapid absorption, rats, given a dosage of 1,450 mg/kg, did not show clear signs of poisoning until 24 hr later, when their brain cholinesterase was inhibited by 46%. Recovery of cholinesterase activity began to be apparent in 72 hr; it was complete for plasma enzyme by 96 hr but was slower for the red cell enzyme. |
| 分子式 |
C11H20N3O3PS
|
|---|---|
| 分子量 |
305.33
|
| 精确质量 |
305.096
|
| CAS号 |
29232-93-7
|
| 相关CAS号 |
Pirimiphos-methyl-d6;1793055-06-7
|
| PubChem CID |
34526
|
| 外观&性状 |
Light yellow to yellow liquid
|
| 密度 |
1.2±0.1 g/cm3
|
| 沸点 |
386.5±52.0 °C at 760 mmHg
|
| 熔点 |
15°C
|
| 闪点 |
187.6±30.7 °C
|
| 蒸汽压 |
0.0±0.9 mmHg at 25°C
|
| 折射率 |
1.555
|
| LogP |
4
|
| tPSA |
98.61
|
| 氢键供体(HBD)数目 |
0
|
| 氢键受体(HBA)数目 |
7
|
| 可旋转键数目(RBC) |
7
|
| 重原子数目 |
19
|
| 分子复杂度/Complexity |
310
|
| 定义原子立体中心数目 |
0
|
| SMILES |
CCN(CC)C1=NC(=CC(=N1)OP(=S)(OC)OC)C
|
| InChi Key |
QHOQHJPRIBSPCY-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C11H20N3O3PS/c1-6-14(7-2)11-12-9(3)8-10(13-11)17-18(19,15-4)16-5/h8H,6-7H2,1-5H3
|
| 化学名 |
4-dimethoxyphosphinothioyloxy-N,N-diethyl-6-methylpyrimidin-2-amine
|
| 别名 |
AI3-27699 Actelic Pirimiphos-methyl
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中(例如氮气保护),避免吸湿/受潮和光照。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~50 mg/mL (~163.76 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (8.19 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: 2.5 mg/mL (8.19 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (8.19 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.2751 mL | 16.3757 mL | 32.7514 mL | |
| 5 mM | 0.6550 mL | 3.2751 mL | 6.5503 mL | |
| 10 mM | 0.3275 mL | 1.6376 mL | 3.2751 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。