| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 10g |
|
||
| Other Sizes |
|
| 靶点 |
CRBN; TNF-α (IC50 = 13 nM)
|
|---|---|
| 体外研究 (In Vitro) |
(放大倍数,×200)。Pomalidomide 抑制人 PBMC 和人全血中脂多糖 (LPS) 刺激的 TNF-α 释放的 IC50 分别为 13 nM 和 25 nM。 [1] Pomalidomide 的 IC50 为 1 μM,抑制 IL-2 诱导的 T 调节细胞生长。 [2] 泊马度胺 (6.4 nM–10 M) 治疗会导致人外周血 T 细胞中 IL-2 的产生增加;这种效应在 CD4+ 子集中比在 CD8+ 子集中稍微更明显。 Pomalidomide 比 CC-5013 具有更强的增加 IL-2、IL-5 和 IL-10 水平的能力,但其增加 IFN-γ 水平的能力仅稍强一些。 Pomalidomide 以剂量依赖性方式增强 SEE 和 Raji 细胞在 Jurkat 细胞中诱导的 AP-1 转录活性,在 1 μM 时最大增强 4 倍。 [3] 当Raji细胞暴露于不同浓度的泊马度胺(2.5-40 μg/mL)48小时时,细胞增殖和DNA合成显着减少。与使用媒介物处理的对照相比,减少了 40%。 [4]
|
| 体内研究 (In Vivo) |
在患有严重联合免疫缺陷的小鼠中,泊马度胺可提高利妥昔单抗治疗 B 细胞淋巴瘤的能力。与CC5013/利妥昔单抗治疗的58天和利妥昔单抗非治疗的45天相比,泊马度胺和利妥昔单抗联合治疗的小鼠的中位生存期为74天。泊马度胺和利妥昔单抗具有协同作用,但这种作用可以通过 NK 细胞耗竭而完全逆转,这支持了以下观点:泊马度胺可能增加利妥昔单抗抗肿瘤活性的一种方式是促进 NK 细胞扩增。 [4]
|
| 酶活实验 |
在脂多糖 (LPS) 刺激的 PBMC 中测量 TNF-α 抑制活性。在添加 LPS (1 μg/mL) 之前一小时,将泊马度胺添加到人 PBMC 中,然后再继续孵育 18 至 20 小时。收获上清液后,使用 ELISA 测量上清液中 TNF-α 的浓度。使用非线性回归分析来确定将 TNF 产量减少 50% 所需的泊马度胺 (IC50) 量。与PBMC测定类似,进行人全血TNF抑制测定,不同之处在于将已肝素化的新鲜人全血直接铺在微量滴定板上。
|
| 细胞实验 |
将泊马度胺 (5 μg/mL) 应用于淋巴瘤细胞系 24 或 48 小时,以测量细胞凋亡。使用碘化丙啶和 FITC 标记的膜联蛋白 V 对细胞进行染色。荧光激活细胞分选仪/FACStar Plus流式细胞仪多色流式细胞分析用于检查细胞凋亡。当细胞表现出早期或晚期凋亡的迹象(分别为膜联蛋白 V 阳性和碘丙啶阴性或阳性)时,它们被认为是凋亡的。将淋巴瘤细胞系暴露于 Pomalidomide(2.5、5、10、20 和 40 μg/mL)24 或 48 小时以测量细胞增殖。每孔添加 1 μCi 的 [3H]-胸苷(在 96 孔板中)后,细胞再孵育 18 小时。使用 Harvest 系统收获细胞并将其放入 96 孔玻璃过滤器后,使用自动闪烁计数器测定 [3H]-胸苷摄取。
|
| 动物实验 |
Mice: SCID mice aged six to eight weeks are used for this. All of the animals are injected with 1×106 Raji cells through their tail veins on day 0. The animals are divided into seven cohorts following 72 hours of tumor engraftment. The first cohort (group A) serves as the control and is not given any medication. Animals in Groups B and C were given either CC-5013 (0.5 mg/kg) or Pomalidomide (0.5 mg/kg) intravenously on Days +3, +4, +8, +9, +13, +14, +18, and +19. Rituximab or Trastuzumab (isotype control) monotherapy is administered to Groups D and E on Days +5, +10, +15, and +20 by tail vein injection at a dose of 10 mg/kg. Animals treated with Rituximab and CC-5013 (group E) or Pomalidomide (group G) make up groups F and G, respectively. Prior to each dose of Rituximab, IMiDs are administered intravenously for two consecutive days. Animals are monitored for 90 days after therapy is over. The study's primary outcome is survival, which is measured as the amount of time before limb paralysis sets in. Cervical dislocation is used to kill any animals that reach the end point or remain alive after three months of observation. To find any remaining disease, a pathologic examination of all organs is conducted, including the liver, lungs, and brain. Three different times, the experiments are repeated.
Rats: Three male CD-IGS rats in total are used. Pomalidomide is given as a single PO administration through the stomach cannula at a dose of 50 mg/kg (5 mL/kg) in a suspension formulation of 0.5% carboxymethylcellulose/0.25% Tween 80. Ten hours after dosing, microdialysate is collected in a cooling fraction collector set to 4°C at intervals of 25 minutes. Each sample's corrected concentration is multiplied by the sampling interval, in this case 25 minutes, and divided by the number of hours in a day to obtain the AUC. These values added together represented the overall AUC value for the given time frame. The concentration is plotted at each time point at the halfway point of each collection interval in order to create graphs. Within 12 hours of the specified time points, microdialysates are collected and analyzed for the presence of pomalidomide using a LC-MS/MS assay. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Pomalidomide is generally well absorbed. The major circulating component is the parent compound. Tmax, single oral dose = 2 -3 hours. When 4 mg of promalidomide is given to patients with multiple myeloma, the steady-state pharmacokinetic parameters are as follows: AUC(T) = 400 ng.hr/mL; Cmax = 75 ng/mL. Promalidomide accumulates following multiple doses. When a single oral dose (2mg) is given to healthy subjects, 73% of the dose was eliminated in urine. 15% of the dose was eliminated in feces. 2% and 8% of the dose eliminated unchanged as pomalidomide in urine and feces, respectively. Mean apparent volume of distribution (Vd/F), steady-state = 62 - 138 L Total body clearance = 7-10 L/hour Pomalidomide has a mean apparent volume of distribution (Vd/F) between 62 and 138 L at steady state. Pomalidomide is distributed in semen of healthy subjects at a concentration of approximately 67% of plasma level at 4 hours post-dose (approximate Tmax) after 4 days of once-daily dosing at 2 mg. Human plasma protein binding ranges from 12% to 44% and is not concentration dependent. Pomalidomide is a substrate for P-glycoprotein (P-gp). In patients with multiple myeloma who received Pomalyst 4 mg daily alone or in combination with dexamethasone, pomalidomide steady-state drug exposure was characterized by AUC(T) of 400 ng*h/mL and Cmax of 75 ng/mL. Following multiple doses, pomalidomide has an accumulation ratio of 27% to 31%. Following a single oral administration of (14)C-pomalidomide (2 mg) to healthy subjects, approximately 73% and 15% of the radioactive dose was eliminated in urine and feces, respectively, with approximately 2% and 8% of the radiolabeled dose eliminated unchanged as pomalidomide in urine and feces. Pomalidomide has a mean total body clearance (CL/F) of 7-10 L/hr. For more Absorption, Distribution and Excretion (Complete) data for Pomalidomide (12 total), please visit the HSDB record page. Metabolism / Metabolites Promalidomide is hepatically metabolized by CYP1A2 and CYP3A4. The metabolites are 26-fold less active than the parent compound. Minor contributions from CYP2C19 and CYP2D6 have been observed in vitro. In hepatocytes from rabbit and human, and in vivo in rat, monkey and human, pomalidomide was metabolized primarily via hydroxylation of the phthalimide ring (M14, M16 and M17) followed by glucuronidation (M12 and M13), hydrolysis of the glutarimide ring (M10 and M11), and hydrolysis of the phthalimide ring (M2). There were no unique or disproportionate metabolites observed in humans, compared to rats and monkeys. Pomalidomide is primarily metabolized in the liver by CYP1A2 and CYP3A4. In vitro, CYP1A2 and CYP3A4 were identified as the primary enzymes involved in the CYP-mediated hydroxylation of pomalidomide, with additional minor contributions from CYP2C19 and CYP2D6. Biological Half-Life Healthy subjects = 9.4 hours; Multiple myeloma patients = 7.5 hours. ... the terminal half-lives of pomalidomide in animals ranged from mean values of 4 to 7 hours following an IV dose. Pomalidomide is eliminated with a median plasma half-life of approximately 9.5 hours in healthy subjects and approximately 7.5 hours in patients with multiple myeloma. |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION AND USE: Pomalidomide is a solid yellow powder. Pomalidomide, a thalidomide analog, is an immunomodulatory agent with antineoplastic and antiangiogenic activity. It is used in patients with multiple myeloma who have received at least two prior therapies including lenalidomide and bortezomib and have demonstrated disease progression on or within 60 days of completion of the last therapy. HUMAN EXPOSURE AND TOXICITY: Pomalidomide may cause fetal toxicity; it is a structural analog of thalidomide, a known human teratogen. Therefore, pomalidomide is contraindicated during pregnancy. Acute myelogenous leukemia (AML) has been reported in patients receiving pomalidomide as investigational therapy for uses other than multiple myeloma. Serious venous thromboembolic events have also been reported in patients receiving pomalidomide. Pomalidomide did not induce chromosomal aberrations in human peripheral blood lymphocytes. ANIMAL STUDIES: Chronic administration of pomalidomide was well tolerated in rats at doses of 50, 250 and 1000 mg/kg/day for 6 months. However, monkeys exhibited greater sensitivity to pomalidomide in the studies reported. The primary toxicities observed in monkeys were associated with the hematopoietic/lymphoreticular systems. In the 9-month study in monkeys with doses of 0.05, 0.1, and 1 mg/kg/day, morbidity and early euthanasia of 6 animals were observed at the dose of 1 mg/kg/day and were attributed to immunosuppressive effects (staphylococcal infection, decreased peripheral blood lymphocytes, chronic inflammation of the large intestine, lymphoid depletion of lymphoid tissues, and lymphoid hypocellularity of bone marrow) at high exposures of pomalidomide. These immunosuppressive effects resulted in early euthanasia of 4 monkeys due to poor health condition (watery stool, inappetence, reduced food intake, and weight loss); histopathological evaluation of these animals showed chronic inflammation of the large intestine and villous atrophy of the small intestine. Staphylococcal infection was observed in 4 monkeys; 3 of these animals responded to antibiotic treatment and 1 died without treatment. In addition, findings consistent with acute myelogenous leukemia led to euthanasia of 1 monkey; clinical observations and clinical pathology and/or bone marrow alterations observed in this animal were consistent with immunosuppression. Minimal or mild bile duct proliferation with associated increases in ALP and GGT were also observed at 1 mg/kg/day. Evaluation of recovery animals indicated that all treatment-related findings were reversible after 8 weeks of dosing cessation, except for proliferation of intrahepatic bile ducts observed in 1 animal in the 1 mg/kg/day group. Pomalidomide was teratogenic in rabbits when administered during the period of major organogenesis. Doses ranging from 10 to 250 mg/kg produced embryo-fetal developmental malformations and variations. Increased cardiac anomalies and skeletal malformations were seen at all dose levels. At 100 and 250 mg/kg/day, there were slight increases in post-implantation loss and slight decreases in fetal body weights. At 100 and/or 250 mg/kg/day, fetal malformations also included limb anomalies and associated skeletal deformities, moderate dilation of the lateral ventricle in the brain, abnormal placement of the right subclavian artery, absent intermediate lobe in the lungs, low-set kidney, altered liver morphology, incompletely or not ossified pelvis, an increased average for supernumerary thoracic ribs and a reduced average for ossified tarsals. Pomalidomide was also teratogenic in rats. Malformations such as the absence of urinary bladder, absence of thyroid gland, and fusion and misalignment of lumbar and thoracic vertebral elements (central and/or neural arches) sometimes associated with discontinuous and misshapen ribs were observed at all dosage levels (25, 250, and 1000 mg/kg/day). In a fertility and early embryonic development study in rats, pomalidomide was administered to male and female rats at doses of 25, 250, and 1000 mg/kg/day before, during, and after mating with animals at the same dose level. Uterine examination on Gestation Day 13 showed a decrease in mean number of viable embryos and an increase in postimplantation loss at all dose levels. Pomalidomide was not mutagenic in bacterial and mammalian mutation Ames assays, and did not induce micronuclei formation in polychromatic erythrocytes in bone marrow of rats administered doses up to 2000 mg/kg/day. Hepatotoxicity Serum enzyme elevations occur in 1% to 2% of patients taking pomalidomide and are more frequent with higher doses. The enzyme abnormalities are usually mild and self-limited and rarely require drug discontinuation. In addition pomalidomide has been implicated in rare instances of clinically apparent, acute liver injury which can be severe and has been reported to lead to deaths from acute liver failure. However, few of these cases have been published and the clinical features, course and outcome of the typical case of liver injury from pomalidomide have not been defined. Both thalidomide and lenalidomide have been implicated in cases of clinically apparent acute liver injury and the presentation and course of injury is likely to be similar to that caused by pomalidomide. The latency to onset of cases of thalidomide associated liver injury is usually within 1 to 6 weeks of starting the antineoplastic agent. The clinical features vary greatly and can be hepatocellular or cholestatic. Cases of acute liver failure as well as vanishing bile duct syndrome with rapid marked cholestasis and hepatic failure have been described with thalidomide and lenalidomide. Immunoallergic features may be prominent and instances of Stevens Johnson syndrome and toxic epidermal necrolysis with and without liver injury have also been linked to therapy with thalidomide and its derivatives. In most cases, the injury resolves rapidly after therapy is stopped. Monitoring of liver tests at monthly intervals is recommended when using thalidomide and its derivatives, and stopping therapy early may play an important role in preventing severe and fatal outcomes. Pomalidomide and the thalidomide derivatives have also been implicated in causing an increased risk of graft-vs-host disease after autologous or allogeneic hematopoietic stem cell transplantation (HSCT) as well as after liver, kidney and heart transplantation. There appears to be cross reactivity to this complication among lenalidomide, pomalidomide and thalidomide. Therapy usually requires discontinuation of the antineoplastic agent as well as treatment with high doses of corticosteroids and tacrolimus or sirolimus. Furthermore, hepatic graft-vs-host disease can occasionally present with an acute hepatitis that resembles hepatocellular drug induced liver injury. Reactivation of hepatitis B has been reported in patients receiving thalidomide, lenalidomide and pomalidomide, but generally only after HSCT and the role of these agents in causing reactivation is not always clear. Indeed, in studies of large numbers of patients treated for multiple myeloma the major risk factor for hepatitis B reactivation was found to be HSCT rather than the specific antineoplastic drugs being used. Indeed, lenalidomide therapy is associated with a reduced risk of reactivation in patients with HSCT (although dexamethasone, thalidomide and bortezomib were not), perhaps because of the immune enhancement typically caused by lenalidomide. Likelihood score: D (possible cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation No information is available on the use of pomalidomide during breastfeeding. The manufacturer recommends that breastfeeding be discontinued during pomalidomide therapy ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding 12-44% protein bound. It is not concentration dependent. Interactions Pomalidomide is a substrate of the efflux transporter P-glycoprotein (P-gp); potent inhibitors or inducers of this transport protein may potentially alter pomalidomide exposure. Concomitant use of pomalidomide with potent inhibitors or inducers of P-gp should be avoided. Metabolism of pomalidomide is mediated primarily by cytochrome P-450 (CYP) isoenzymes 1A2 and 3A4. Concomitant use of pomalidomide with potent inhibitors of CYP1A2 or CYP3A (e.g., ketoconazole) may increase exposure to pomalidomide and should be avoided. Conversely, concomitant use of pomalidomide with potent inducers of CYP1A2 (e.g., cigarette smoking) or CYP3A (e.g., rifampin) may decrease exposure to pomalidomide and also should be avoided. When the weak CYP3A inducer dexamethasone (20-40 mg once daily) was administered concomitantly with pomalidomide (4 mg once daily) in patients with multiple myeloma, pharmacokinetics of pomalidomide were unchanged. Pomalidomide does not inhibit or induce CYP isoenzymes in vitro. Thalidomide and the immunomodulatory drug, lenalidomide, are therapeutically active in hematological malignancies. The ubiquitously expressed E3 ligase protein cereblon (CRBN) has been identified as the primary teratogenic target of thalidomide. Our studies demonstrate that thalidomide, lenalidomide and another immunomodulatory drug, pomalidomide, bound endogenous CRBN and recombinant CRBN-DNA damage binding protein-1 (DDB1) complexes. CRBN mediated antiproliferative activities of lenalidomide and pomalidomide in myeloma cells, as well as lenalidomide- and pomalidomide-induced cytokine production in T cells. Lenalidomide and pomalidomide inhibited autoubiquitination of CRBN in HEK293T cells expressing thalidomide-binding competent wild-type CRBN, but not thalidomide-binding defective CRBN(YW/AA). Overexpression of CRBN wild-type protein, but not CRBN(YW/AA) mutant protein, in KMS12 myeloma cells, amplified pomalidomide-mediated reductions in c-myc and IRF4 expression and increases in p21(WAF-1) expression. Long-term selection for lenalidomide resistance in H929 myeloma cell lines was accompanied by a reduction in CRBN, while in DF15R myeloma cells resistant to both pomalidomide and lenalidomide, CRBN protein was undetectable. Our biophysical, biochemical and gene silencing studies show that CRBN is a proximate, therapeutically important molecular target of lenalidomide and pomalidomide. Pomalidomide offers an alternative for patients with relapsed/refractory multiple myeloma who have exhausted treatment options with lenalidomide and bortezomib. Little is known about pomalidomide's potential for drug-drug interactions (DDIs); as pomalidomide clearance includes hydrolysis and cytochrome P450 (CYP450)-mediated hydroxylation, possible DDIs via CYP450 and drug-transporter proteins were investigated in vitro and in a clinical study. In vitro pomalidomide was neither an inducer nor inhibitor of CYP450, nor an inhibitor of transporter proteins P glycoprotein (P-gp), BCRP, OAT1, OAT3, OCT2, OATP1B1, and OATP1B3. Oxidative metabolism of pomalidomide was predominately mediated by CYP1A2 and CYP3A4, and pomalidomide was shown to be a P-gp substrate. In healthy males, co-administration of oral (4 mg) pomalidomide with ketoconazole (CYP3A/P-gp inhibitor) or carbamazepine (CYP3A/P-gp inducer) did not result in clinically relevant changes in pomalidomide exposure. Co-administration of pomalidomide with fluvoxamine (CYP1A2 inhibitor) in the presence of ketoconazole approximately doubled pomalidomide exposure. Pomalidomide appears to have low potential for clinically relevant DDI and is unlikely to affect the clinical exposure of other drugs. Avoid co-administration of strong CYP1A2 inhibitors unless medically necessary. Pomalidomide dose should be reduced by 50% if co-administered with strong CYP1A2 inhibitors and strong CYP3A/P-gp inhibitors. |
| 参考文献 |
|
| 其他信息 |
Therapeutic Uses
Angiogenesis Inhibitors; Immunologic Factors Pomalyst is indicated for patients with multiple myeloma who have received at least two prior therapies including lenalidomide and bortezomib and have demonstrated disease progression on or within 60 days of completion of the last therapy. Approval is based on response rate. Clinical benefit, such as improvement in survival or symptoms, has not been verified. /Included in US product label/ EXPL THER Pomalidomide /affected/ the regulation of fetal hemoglobin (HbF) making it a potential therapeutic agent for the treatment of non-malignant hematologic disorders such as sickle cell disease (SCD) and beta-thalassemia. In vitro pomalidomide was a more potent inducer of HbF than hydroxyurea (HU), the only treatment currently approved for SCD. Pomalidomide increased the expression of genes directing the production of HbF as well as gamma- and epsilon-globin gene transcription and expression during erythroid differentiation. In an in vivo knockout transgenic mouse model of SCD, pomalidomide (10 mg/kg; 5 QD/week x 8) stimulated erythropoiesis as indicated by bone marrow hyperplasia and increased extramedullary hematopoiesis, a trend toward higher reticulocytes and significantly higher red blood cell (RBC) levels. Pomalidomide significantly increased HbF expression with a trend toward higher gamma-globin chain A levels. The pomalidomide responder rate, defined as the percentage of animals that exceeded the maximum HbF and gamma-globin chain A levels in the vehicle group, reached 67% and 78% respectively. Among responders, pomalidomide induced a nearly 2-fold increase in HbF and the increase in gamma-globin chain A levels was significant and was similar to the approved HbF-inducing agent HU. Drug Warnings /BOXED WARNING/ WARNING: EMBRYO-FETAL TOXICITY. Embryo-Fetal Toxicity: Pomalyst is contraindicated in pregnancy. Pomalyst is a thalidomide analogue. Thalidomide is a known human teratogen that causes severe birth defects or embryo-fetal death. In females of reproductive potential, obtain 2 negative pregnancy tests before starting Pomalyst treatment. Females of reproductive potential must use 2 forms of contraception or continuously abstain from heterosexual sex during and for 4 weeks after stopping Pomalyst treatment. Pomalyst is only available through a restricted distribution program called Pomalyst REMS. /BOXED WARNING/ WARNING: VENOUS THROMBOEMBOLISM. Deep venous thrombosis (DVT) and pulmonary embolism (PE) occur in patients with multiple myeloma treated with Pomalyst. Prophylactic anti-thrombotic measures were employed in the clinical trial. Consider prophylactic measures after assessing an individual patient's underlying risk factors Use of pomalidomide should be avoided in patients with serum aminotransferase (ALT and AST) concentrations exceeding 3 times the upper limit of normal (ULN) and bilirubin concentrations exceeding 2 mg/dL. Use of pomalidomide also should be avoided in patients with serum creatinine concentrations exceeding 3 mg/dL. Safety and efficacy have not been established in these patients. Pomalidomide may cause fetal toxicity; pomalidomide is a structural analog of thalidomide, a known human teratogen, and teratogenic and other fetotoxic effects of pomalidomide (e.g., musculoskeletal anomalies and deformities; absence of internal organs, including bladder and thyroid; defects of internal organ systems, including cardiovascular, respiratory, renal, hepatic, and CNS abnormalities; increased fetal resorptions) have been demonstrated in animals. Therefore, pomalidomide is contraindicated in women who are pregnant. For more Drug Warnings (Complete) data for Pomalidomide (21 total), please visit the HSDB record page. Pharmacodynamics Pomalidomide is more potent than thalidomide (100-times) and lenalidomide (10-times). |
| 分子式 |
C13H11N3O4
|
|---|---|
| 分子量 |
273.24
|
| 精确质量 |
273.074
|
| 元素分析 |
C, 57.14; H, 4.06; N, 15.38; O, 23.42
|
| CAS号 |
19171-19-8
|
| 相关CAS号 |
Pomalidomide-d3;2093128-28-8;Pomalidomide-d5;1377838-49-7;Pomalidomide-d4;1416575-78-4
|
| PubChem CID |
134780
|
| 外观&性状 |
white solid powder
|
| 密度 |
1.6±0.1 g/cm3
|
| 沸点 |
582.9±45.0 °C at 760 mmHg
|
| 熔点 |
318.5 - 320.5°
|
| 闪点 |
306.3±28.7 °C
|
| 蒸汽压 |
0.0±1.6 mmHg at 25°C
|
| 折射率 |
1.691
|
| LogP |
-0.74
|
| tPSA |
109.57
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
5
|
| 可旋转键数目(RBC) |
1
|
| 重原子数目 |
20
|
| 分子复杂度/Complexity |
504
|
| 定义原子立体中心数目 |
0
|
| SMILES |
O=C1C([H])(C([H])([H])C([H])([H])C(N1[H])=O)N1C(C2C([H])=C([H])C([H])=C(C=2C1=O)N([H])[H])=O
|
| InChi Key |
UVSMNLNDYGZFPF-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C13H11N3O4/c14-7-3-1-2-6-10(7)13(20)16(12(6)19)8-4-5-9(17)15-11(8)18/h1-3,8H,4-5,14H2,(H,15,17,18)
|
| 化学名 |
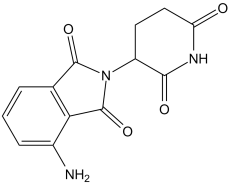
4-amino-2-(2,6-dioxopiperidin-3-yl)isoindole-1,3-dione
|
| 别名 |
CC4047; CC-4047; CC 4047; Pomalidomide. Brand name: Pomalyst
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (9.15 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (9.15 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL 澄清 DMSO 储备液添加到 900 μL 玉米油中并混合均匀。 View More
配方 3 中的溶解度: 1% DMSO +30% polyethylene glycol+1% Tween 80 : 15mg/mL 配方 4 中的溶解度: 10 mg/mL (36.60 mM) in 0.5% CMC-Na 0.5% Tween-80 (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.6598 mL | 18.2989 mL | 36.5979 mL | |
| 5 mM | 0.7320 mL | 3.6598 mL | 7.3196 mL | |
| 10 mM | 0.3660 mL | 1.8299 mL | 3.6598 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Pomalidomide in Combination With Liposomal Doxorubicin in People With Advanced or Refractory Kaposi Sarcoma
CTID: NCT02659930
Phase: Phase 1 Status: Active, not recruiting
Date: 2024-11-25