| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
Pralatrexate (racemic) primarily targets dihydrofolate reductase (DHFR, Ki = 0.015 μM) and thymidylate synthase (TS, Ki = 0.12 μM), two key folate-dependent enzymes in nucleotide biosynthesis. These Ki values were determined via in vitro enzyme inhibition assays using purified human enzymes [4]
- Pralatrexate (racemic) inhibits DHFR to block tetrahydrofolate production, thereby suppressing nucleotide synthesis [1] |
|---|---|
| 体外研究 (In Vitro) |
当给予不同的 T 淋巴瘤细胞系时,普拉曲沙(100 pM-200 μM;48-72 小时)表现出取决于浓度和时间的细胞毒性。以下是 48 小时和 72 小时的 IC50 值:H9 细胞为 1.1 和 2.5 nM; P12 细胞为 1.7 和 2.4 nM; CEM 细胞为 3.2 和 4.2 nM; PF-382 细胞为 5.5 和 2.7 nM; KOPT-K1 细胞为 1 和 1.7 nM; DND-41 细胞为 97.4 和 1.2 nM; HPB-ALL 细胞为 247.8 nM 和 0.77 nM。治疗 48 小时后,HH 细胞表现出一定的耐药性,72 小时时的 IC50 为 2.8 nM [1]。用 pralidoxate(2-5.5 nM;48-72 小时;H9、HH、P12 和 PF382 细胞)处理会导致强烈的细胞凋亡以及 caspase-8 和 caspase-9 激活 [1]。用普拉曲沙(3 nM;16-48 小时)处理 H9 和 P12 细胞可显着提高 p27 水平,并促进诱导型叶酸载体 1 型 (RFC-1) 在细胞中的积累 [1]。
在人T细胞淋巴瘤细胞系(Jurkat、Karpas 299、Hut-78)中:单独使用普拉曲沙(外消旋体)的抗增殖活性IC50值为:Jurkat细胞0.008 μM、Karpas 299细胞0.012 μM、Hut-78细胞0.015 μM(MTT法,孵育72小时)。与硼替佐米(0.005 μM)联用时,联合指数(CI)为0.4-0.6(CI < 1提示协同作用),凋亡细胞(Annexin V阳性)比例从单药组的15%-20%升至联合组的45%-55%。Western blot显示,联合用药使胱天蛋白酶-3(caspase-3)和多聚ADP核糖聚合酶(PARP)的切割水平(凋亡标志物)较单独使用普拉曲沙(外消旋体)提高2-3倍 [1] - 在人实体瘤细胞系(HCT-116结直肠癌、A549非小细胞肺癌、MDA-MB-231乳腺癌)中:普拉曲沙(外消旋体)抑制细胞活力的IC50值为:HCT-116细胞0.006 μM、A549细胞0.009 μM、MDA-MB-231细胞0.011 μM(MTT法,孵育72小时)。PCR分析显示,0.01 μM 普拉曲沙(外消旋体)使HCT-116细胞中TS mRNA表达下调40%-50% [4] |
| 体内研究 (In Vivo) |
与单独使用任一药物相比,硼替佐米 (0.5 mg/kg) 和普拉曲沙 (15 mg/kg;腹腔注射;第 1、4、8 和 11 天;SCID-米色小鼠) 组合可提高疗效 [1]。
携带Karpas 299(T细胞淋巴瘤)异种移植物的裸鼠:将小鼠分为4组(每组n=6):对照组(溶媒)、普拉曲沙(外消旋体)单药组(10 mg/kg,腹腔注射,每周2次)、硼替佐米单药组(0.5 mg/kg,腹腔注射,每周2次)、联合用药组。治疗3周后,肿瘤体积抑制率分别为:普拉曲沙(外消旋体)单药45%-50%、硼替佐米单药30%-35%、联合用药75%-80%。肿瘤组织免疫组化显示,联合用药使Ki-67(增殖标志物)阳性细胞比例降低60%-70%,而普拉曲沙(外消旋体)单药仅降低30%-35% [1] - 携带HCT-116(结直肠癌)或MDA-MB-231(乳腺癌)异种移植物的裸鼠:普拉曲沙(外消旋体)以15 mg/kg剂量静脉注射,每周1次,持续4周。肿瘤重量抑制率为:HCT-116细胞异种移植物60%-65%、MDA-MB-231细胞异种移植物55%-60%。治疗组小鼠血清胸苷(TS底物)水平降低50%-55%,证实其在体内抑制TS活性 [4] - PROPEL研究(II期临床):对复发/难治性转化型蕈样肉芽肿患者给予普拉曲沙(外消旋体)(30 mg/m²,静脉输注,每周1次,连续6周后休息2周)治疗。总缓解率(ORR)为28%(95%置信区间:16%-43%),中位无进展生存期(PFS)为4.5个月。仅皮肤受累患者的ORR为35%,高于皮肤外受累患者(18%) [2] - 随机IIb期研究(铂类治疗失败的非小细胞肺癌患者):患者分别接受普拉曲沙(外消旋体)(60 mg/m²,静脉输注,每2周1次)或厄洛替尼(150 mg/天,口服)治疗。中位PFS为:普拉曲沙组2.6个月 vs. 厄洛替尼组2.8个月(p=0.78);ORR为:普拉曲沙组5% vs. 厄洛替尼组8%。两组疗效无显著差异 [3] |
| 酶活实验 |
DHFR活性检测:反应在含10 mM MgCl2、0.1 mM NADPH和0.05 mM二氢叶酸(底物)的50 mM Tris-HCl缓冲液(pH 7.4)中进行。将纯化人源DHFR与普拉曲沙(外消旋体)(0.001-0.1 μM)在37°C预孵育10分钟,加入底物启动反应。每分钟记录340 nm处吸光度(因NADPH氧化导致吸光度下降),持续20分钟。通过双倒数作图法(Lineweaver-Burk plot)拟合抑制曲线,计算Ki值 [4]
- TS活性检测:反应体系为含5 mM MgCl2、0.1 mM [3H]-dUMP(底物)和0.2 mM N5,N10-亚甲基四氢叶酸(辅酶)的50 mM磷酸钾缓冲液(pH 7.2)。将纯化人源TS与普拉曲沙(外消旋体)(0.01-1 μM)在37°C孵育15分钟,加入底物。30分钟后,用10%三氯乙酸终止反应,通过液体闪烁计数法检测放射性沉淀([3H]-dTMP)的量。根据TS活性的剂量依赖性抑制效应,计算Ki值 [4] |
| 细胞实验 |
细胞毒性测定[1]
细胞类型: T 淋巴瘤细胞系 测试浓度: 100 pM-200 µM 孵育时间: 48 小时、72 小时 实验结果: 证明对多种 T 淋巴瘤细胞系具有浓度和时间依赖性细胞毒性。 细胞凋亡分析[1] 细胞类型: H9、HH、P12 和 PF382 细胞 测试浓度: 2 nM、3 nM , 4 nM, 5.5 nM 孵育时间: 48 小时、72 小时 实验结果: 诱导有效的细胞凋亡和 caspase 激活。 蛋白质印迹分析[1] 细胞类型: H9 和 P12 细胞 测试浓度: 3 nM 孵化持续时间:16小时、24小时、48小时 实验结果:明显增加p27水平并增加RFC-1的积累细胞。 MTT抗增殖实验(T细胞淋巴瘤细胞):将Jurkat、Karpas 299和Hut-78细胞以2×10⁴个/孔的密度接种于96孔板,使用含10%胎牛血清的RPMI 1640培养基培养。加入浓度为0.001-0.1 μM的普拉曲沙(外消旋体),在37°C、5% CO2条件下孵育72小时。每孔加入20 μL MTT溶液(5 mg/mL PBS),继续孵育4小时。去除上清液,加入150 μL二甲基亚砜溶解甲臜结晶,检测570 nm处吸光度。将抑制50%细胞活力的药物浓度定义为IC50 [1] - Annexin V/PI凋亡检测(Karpas 299细胞):将细胞以1×10⁶个/孔接种于6孔板,用普拉曲沙(外消旋体)(0.01 μM)单药或联合硼替佐米(0.005 μM)处理48小时。收集细胞,用冷PBS洗涤,重悬于结合缓冲液中。加入Annexin V-FITC和PI,室温避光孵育20分钟,通过流式细胞术分析凋亡细胞比例 [1] - 克隆形成实验(HCT-116细胞):将细胞以100个/孔接种于6孔板,贴壁24小时后,加入0.002、0.005、0.01 μM的普拉曲沙(外消旋体),培养14天(每3天换液一次)。用4%多聚甲醛固定克隆15分钟,0.1%结晶紫染色30分钟,计数含50个以上细胞的克隆。克隆形成率=(治疗组克隆数/对照组克隆数)×100% [4] |
| 动物实验 |
Animal/Disease Models: SCID-beige mice (5-7weeks old) injected with HH cells[1]
Doses: 15 mg/kg Route of Administration: intraperitoneal (ip)injection; on days 1, 4, 8, and 11 Experimental Results: demonstrated superior efficacy in T-cell malignancies. Karpas 299 Xenograft Model (nude mice): Female nude mice (6-8 weeks old, 18-22 g) were subcutaneously injected with 2×106 Karpas 299 cells (suspended in 0.2 mL PBS/Matrigel 1:1) into the right flank. When tumors reached 100-150 mm³, mice were randomized into 4 groups: (1) Control: 0.9% saline + 0.1% dimethyl sulfoxide (intraperitoneal injection, twice weekly); (2) Pralatrexate (racemic): dissolved in 0.9% saline + 0.1% dimethyl sulfoxide, 10 mg/kg, intraperitoneal injection, twice weekly; (3) Bortezomib: dissolved in 0.9% saline, 0.5 mg/kg, intraperitoneal injection, twice weekly; (4) Combination: same doses and schedule as single drugs. Tumor volume (length × width² / 2) and body weight were measured twice weekly for 3 weeks. At study end, mice were euthanized, and tumors were collected for immunohistochemistry [1] - HCT-116/MDA-MB-231 Xenograft Models (nude mice): Male nude mice (6-8 weeks old, 20-24 g) were subcutaneously implanted with 5×106 HCT-116 or 3×106 MDA-MB-231 cells (0.2 mL PBS/Matrigel 1:1) into the left flank. When tumors grew to 200-250 mm³, mice were divided into 2 groups (n=5/group): control (5% glucose solution) and Pralatrexate (racemic) (dissolved in 5% glucose solution, 15 mg/kg, intravenous injection, once weekly). Treatment lasted 4 weeks, with tumor volume and body weight measured weekly. Mice were sacrificed at study end, and tumors/serum were collected for analysis [4] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
With an intravenous formulation, pralatrexate has complete bioavailability. Pralatrexate demonstrates a dose-proportional and linear pharmacokinetics over a dose range of 30-325 mg/m2. Upon an intravenous push over 3 to 5 minutes of a starting dose of 30 mg/m2 racemic pralatrexate for dose 1 of cycle 1, Cmax and AUC0-∞ was estimated to be 5,815 ng/mL and 267,854 ng/mL.min respectively using a noncomparmental pharmacokinetics analysis.Both pralatrexate diastereomers demonstrates a multiphase decline in plasma concentration with a rapid initial fall followed by a slow terminal phase. The initial fall is thought to reflect the clearance of pralatrexate by renal and non-renal mechanism , while the slow terminal phase likely represents the return of pralatrexate from deep intracellular compartments, enterohepatic circulation, or after deglutamination. Following a single dose of FOLOTYN 30 mg/m 2 , approximately 34% of the pralatrexate dose was excreted unchanged into urine. Following a radiolabeled pralatrexate dose, 39% (CV = 28%) of the dose was recovered in urine as unchanged pralatrexate and 34% (CV = 88%) in feces as unchanged pralatrexate and/or any metabolites. 10% (CV = 95%) of the dose was exhaled over 24 hours. The steady-state volume of distribution of pralatrexate S- and R-diastereomers is 105 L and 37 L, respectively. The total systemic clearance of pralatrexate diastereomers was 417 mL/min (S-diastereomer) and 191 mL/min (R-diastereomer). The pharmacokinetics of pralatrexate administered as a single agent at a dose of 30 mg/sq m administered as an intravenous push over 3-5 minutes once weekly for 6 weeks in 7-week cycles have been evaluated in 10 patients with PTCL. The total systemic clearance of pralatrexate diastereomers was 417 mL/min (S-diastereomer) and 191 mL/min (R-diastereomer). Pralatrexate total systemic exposure (AUC) and maximum plasma concentration (Cmax) increased proportionally with dose (dose range 30-325 mg/sq m, including pharmacokinetics data from high dose solid tumor clinical studies). The pharmacokinetics of pralatrexate did not change significantly over multiple treatment cycles, and no accumulation of pralatrexate was observed. Pralatrexate diastereomers showed a steady-state volume of distribution of 105 L (S-diastereomer) and 37 L (R-diastereomer). In vitro studies indicate that pralatrexate is approximately 67% bound to plasma proteins. For more Absorption, Distribution and Excretion (Complete) data for Pralatrexate (7 total), please visit the HSDB record page. Metabolism / Metabolites While the liver has been shown to metabolize pralatrexate to some extent, pralatrexate is not significantly metabolized by any CYP450 isozymes or glucuronidases in vitro. In vitro studies using human hepatocytes, liver microsomes and S9 fractions, and recombinant human CYP450 isozymes showed that pralatrexate is not significantly metabolized by the phase I hepatic CYP450 isozymes or phase II hepatic glucuronidases. Biological Half-Life The terminal elimination half-life of pralatrexate was 12-18 hours (coefficient of variance [CV] = 62-120%). The terminal elimination half-life of pralatrexate was 12-18 hours (coefficient of variance (CV) = 62-120%). In nude mice treated with Pralatrexate (racemic) (15 mg/kg, intravenous injection): The plasma concentration-time curve followed a two-compartment model. The terminal half-life (t1/2β) was 2.8 ± 0.3 hours, AUC0-∞ was 18.5 ± 2.1 μg·h/mL, and clearance (CL) was 0.8 ± 0.1 mL/h/g. Approximately 70%-75% of the dose was excreted unchanged in urine within 24 hours [4] - In patients with transformed mycosis fungoides (PROPEL study): After intravenous infusion of Pralatrexate (racemic) (30 mg/m²), the mean plasma half-life was 1.8 ± 0.5 hours, and the volume of distribution at steady state (Vdss) was 12.6 ± 3.2 L/m². The drug was primarily eliminated via renal excretion, with 65%-70% of the dose recovered in urine within 48 hours [2] |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
Pralatrexate is associated with serum enzyme elevations during therapy, but these abnormalities are generally mild and self-limited, rising to above 5 times ULN in 2% to 6% of patients and rarely requiring dose adjustment. No instances of clinically apparent acute liver injury attributed to pralatrexate have been reported in the literature, but monitoring for liver toxicity is recommended. Pralatrexate has not been linked specifically to sinusoidal obstruction syndrome, but it is rarely used in high doses in neoplastic disease or in conditioning regimens for bone marrow transplantation, situations in which alkylating agents are commonly associated with this complication. Likelihood score: E (unlikely but suspected rare cause of liver injury). Protein Binding The protein binding of pralatrexate is approximately 67% in vitro. Interactions The effect of co-administration of the uricosuric drug probenecid on pralatrexate pharmacokinetics was investigated in a Phase 1 clinical study. Co-administration of increasing doses of probenecid resulted in delayed clearance of pralatrexate and a commensurate increase in exposure. Due to the contribution of renal excretion (approximately 34%) to the overall clearance of pralatrexate, concomitant administration of drugs that are subject to substantial renal clearance (eg, NSAIDs, trimethoprim/sulfamethoxazole) may result in delayed clearance of pralatrexate. In nude mice (Karpas 299 model): Pralatrexate (racemic) (10 mg/kg, twice weekly) caused a transient 8%-10% body weight loss in the first week, which recovered by week 2. No significant changes in serum ALT, AST, or creatinine were observed compared to control [1] - In the PROPEL study (patients): The most common grade 3/4 adverse events (AEs) of Pralatrexate (racemic) were mucositis (25%), thrombocytopenia (20%), and neutropenia (15%). Grade 1/2 AEs included fatigue (40%), nausea (30%), and diarrhea (20%). The plasma protein binding rate of Pralatrexate (racemic) was 90%-95% (measured in human plasma in vitro) [2] - In the phase 2b NSCLC study: Grade 3/4 AEs of Pralatrexate (racemic) (60 mg/m², every 2 weeks) included anemia (18%), mucositis (12%), and elevated liver enzymes (10%) [3] - In nude mice (HCT-116 model): Pralatrexate (racemic) (15 mg/kg, weekly) did not cause significant organ toxicity (histological examination of liver/kidney showed no abnormal lesions) [4] |
| 参考文献 |
|
| 其他信息 |
Therapeutic Uses
Aminopterin/ analogs & derivatives; Folic Acid Antagonists Pralatrexate is indicated for the treatment of patients with relapsed or refractory peripheral T-cell lymphoma (PTCL). This indication is based on overall response rate. Clinical benefit such as improvement in progression free survival or overall survival has not been demonstrated. /Included in US product label/ T-cell lymphomas (TCL) are characterized by poor response to chemotherapy and generally poor outcome. While molecular profiling has identified distinct biological subsets and therapeutic targets in B-cell lymphomas, the molecular characterization of TCL has been slower. Surface markers expressed on malignant T-cells, such as CD2, CD3, CD4, CD25, and CD52 were the first TCL-specific therapeutic targets to be discovered. However, the presence of these receptors on normal T-cells means that monoclonal antibody (mAb)- or immunotoxin (IT)-based therapy in TCL inevitably results in variable degrees of immunosuppression. Thus, although some mAbs/IT have significant activity in selected subsets of TCL, more specific agents that target signaling pathways preferentially activated in malignant T-cells are needed. One such novel class of agents is represented by the histone deacetylase (HDAC) inhibitors. These molecules selectively induce apoptosis in a variety of transformed cells, including malignant T-cells, both in vitro and in vivo. Several HDAC inhibitors have been studied in TCL with promising results, and have recently been approved for clinical use. Immunomodulatory drugs, such as interferons and Toll Receptor (TLR) agonists have significant clinical activity in TCL, and are particularly important in the treatment of primary cutaneous subtypes (CTCL). Although most classical cytotoxic drugs have limited efficacy against TCL, agents that inhibit purine and pyrimidine metabolism, known as nucleoside analogues, and novel antifolate drugs, such as pralatrexate, are highly active in TCL. With improved molecular profiling of TCL novel pharmacological agents with activity in TCL are now being discovered at an increasingly rapid pace. Clinical trials are in progress and these agents are being integrated in combination therapies for TCL, both in the relapsed/refractory setting as well as front line. Drug Warnings FOLOTYN can suppress bone marrow function, manifested by thrombocytopenia, neutropenia, and anemia. Dose modifications are based on ANC and platelet count prior to each dose. Treatment with FOLOTYN may cause mucositis. If /greater than or equal to/ Grade 2 mucositis is observed, dose should be modified. Patients should be instructed to take folic acid and receive vitamin B12 to potentially reduce treatment-related hematological toxicity and mucositis. ... Patients should take low-dose oral folic acid on a daily basis. Folic acid should be initiated during the 10-day period preceding the first dose of FOLOTYN, and dosing should continue during the full course of therapy and for 30 days after the last dose of FOLOTYN. Patients should also receive a vitamin B12 intramuscular injection no more than 10 weeks prior to the first dose of FOLOTYN and every 8-10 weeks thereafter. Subsequent vitamin B12 injections may be given the same day as treatment with FOLOTYN. Although FOLOTYN has not been formally tested in patients with renal impairment, caution is advised when administering FOLOTYN to patients with moderate to severe impairment. Monitor patients for renal function and systemic toxicity due to increased drug exposure. For more Drug Warnings (Complete) data for Pralatrexate (10 total), please visit the HSDB record page. Pharmacodynamics Pralatrexate is a folate analog that inhibits folate metabolism, thus impeding the synthesis of amino acids and nucleic acid. Additionally, pralatrexate also competes for enzymatic processing by folyopolyglutamate synthase (FPGS)with folate to increase cellular retention. Compared to methotrexate, pralatrexate binds to the reduced folate carrier protein-1 (RFC-1) for cellular uptake with 10-times the affinity and is a more potent substrate for FPGS. The Km value for RFC-1 was calculated to be 0.3 μmol/L and 4.8 μmol/L for pralatrexate and methotrexate respectively, while the Km value for FPGS was estimated to be 5.9 and 32.3 µmol/l for pralatrexate and methotrexate respectively. As a result, pralatrexate is more cytotoxic and better retained in cancer cells. Due to its anti-folate activity, pralatrexate's main toxicity is manifested as mucositis that can require dose interruption or reduction. In 5 patients with non-small-cell lung carcinoma receiving a supratherapeutic dose of 230 mg/m 2 , the mean change from pre-injection QTcF interval at the end of infusion was 6.1 ms (90%CI: -0.6, 12.7), and at 1-hour post-injection was 7.8 ms (90%CI: 3.0, 12.6). However, no patient exceeded a QTcF of 470 msec and exhibited an absolute increase from baseline in QTcF exceeding 30 msec. As well, the study dose far exceeded the target dose for patients with peripheral T-cell lymphoma and pralatrexate does not inhibit the human ether-a-go-go-related gene (hERG) K + channel. Therefore, pralatrexate uses are unlikely to cause cardiac repolarization delays.. Pralatrexate (racemic) exerts synergistic anti-tumor effects with bortezomib in T-cell lymphoma by enhancing apoptosis: bortezomib inhibits proteasomal degradation of pro-apoptotic proteins, while Pralatrexate (racemic) suppresses nucleotide synthesis, creating a "metabolic stress + apoptotic sensitization" effect [1] - The PROPEL study confirmed that Pralatrexate (racemic) is a valid treatment option for relapsed/refractory transformed mycosis fungoides, especially in patients with skin-only disease (higher ORR). Prophylactic folate and vitamin B12 supplementation were used in the study to reduce mucositis and myelosuppression [2] - In the phase 2b NSCLC study, Pralatrexate (racemic) showed similar efficacy to erlotinib in post-platinum patients but had a different AE profile (more mucositis vs. erlotinib’s rash/diarrhea), suggesting it may be an alternative for patients intolerant to EGFR inhibitors [3] - Pralatrexate (racemic) is a structural analogue of 10-deazaaminopterin with enhanced affinity for DHFR (10-fold higher than methotrexate) and improved tumor penetration, contributing to its higher in vivo efficacy against human xenografts [4] |
| 分子式 |
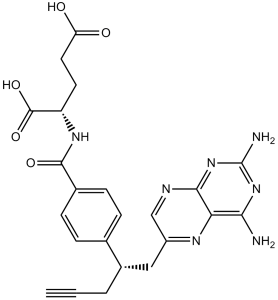
C23H23N7O5
|
|---|---|
| 分子量 |
477.47
|
| 精确质量 |
477.176
|
| CAS号 |
146464-95-1
|
| 相关CAS号 |
(R)-Pralatrexate;1320211-70-8
|
| PubChem CID |
148121
|
| 外观&性状 |
Light yellow to yellow solid powder
|
| 密度 |
1.5±0.1 g/cm3
|
| 熔点 |
215 °C(dec.)
|
| 折射率 |
1.704
|
| LogP |
0.23
|
| tPSA |
207.3
|
| 氢键供体(HBD)数目 |
5
|
| 氢键受体(HBA)数目 |
11
|
| 可旋转键数目(RBC) |
10
|
| 重原子数目 |
35
|
| 分子复杂度/Complexity |
809
|
| 定义原子立体中心数目 |
1
|
| SMILES |
C#CCC(CC1=CN=C2C(=N1)C(=NC(=N2)N)N)C3=CC=C(C=C3)C(=O)N[C@@H](CCC(=O)O)C(=O)O
|
| InChi Key |
OGSBUKJUDHAQEA-WMCAAGNKSA-N
|
| InChi Code |
InChI=1S/C23H23N7O5/c1-2-3-14(10-15-11-26-20-18(27-15)19(24)29-23(25)30-20)12-4-6-13(7-5-12)21(33)28-16(22(34)35)8-9-17(31)32/h1,4-7,11,14,16H,3,8-10H2,(H,28,33)(H,31,32)(H,34,35)(H4,24,25,26,29,30)/t14?,16-/m0/s1
|
| 化学名 |
N -(4-{1-[(2,4-diaminopteridin-6-yl)methyl]but-3-yn-1-yl}benzoyl)-L-glutamic acid
|
| 别名 |
PDX; Pralatrexate; 10-Propargyl-10-deazaaminopterin; trade name: Folotyn.
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (5.24 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (5.24 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.0944 mL | 10.4719 mL | 20.9437 mL | |
| 5 mM | 0.4189 mL | 2.0944 mL | 4.1887 mL | |
| 10 mM | 0.2094 mL | 1.0472 mL | 2.0944 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT02594267 | Completed | Drug: Pralatrexate Injection | Peripheral T-Cell Lymphoma (PTCL) | Acrotech Biopharma Inc. | November 10, 2015 | Phase 1 |
| NCT03355768 | Withdrawn | Drug: Romidepsin Drug: Pralatrexate |
Lymphoma, T-Cell, Peripheral | Jennifer Amengual | September 1, 2018 | Phase 3 |
| NCT03598998 | Active, not recruiting | Biological: Pembrolizumab Drug: Pralatrexate |
Anaplastic Large Cell Lymphoma Nodal Peripheral T-Cell Lymphoma With TFH Phenotype |
City of Hope Medical Center | February 4, 2019 | Phase 1 Phase 2 |
| NCT03240211 | Recruiting | Drug: Pembrolizumab Drug: Pralatrexate |
PTCL CTCL |
University of Virginia | February 2, 2022 | Phase 1 |
 |
|---|
 |
 |