| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1g |
|
||
| 2g |
|
||
| 5g |
|
||
| 10g |
|
||
| 25g |
|
||
| 50g |
|
||
| 100g |
|
||
| 200g |
|
| 靶点 |
Resins for ion-exchange chromatography
|
|---|---|
| 体外研究 (In Vitro) |
PNLP的分离纯化[2]
按照Zhang等人[Carbohydr.Polym.2021251117078]和Li等人[Int.J.Mol.Sci.2023,2415904]报道的方法分离和纯化PNLP。将10mg/mL多糖溶液沿着DEAE纤维素柱(26mm×50cm)的壁缓慢施加。然后依次用蒸馏水和浓度为0.1、0.3和0.5 M的NaCl溶液洗脱样品。洗脱以0.5 mL/min的流速进行,每管以12分钟的间隔收集洗脱液。使用苯酚-硫酸测定法在490nm处定量吸光度,随后绘制所得洗脱曲线。根据洗脱曲线,获得了四个不同的组分:PNLP-1(用蒸馏水洗脱)、PNLP-2(用0.1 M NaCl洗脱)、PN LP-3(用0.3 M NaCl洗脱”)和PNLP-4(用0.5 M NaCl洗脱“)。 竹笋多糖的分离纯化[3] 通过热水提取法从竹笋中提取粗多糖,然后进行脱蛋白、水透析、DEAE纤维素和Sephadex-50柱层析分级处理。结果表明,木瓜蛋白酶与Sevag的结合是最佳的脱蛋白条件。采用DEAE-Cellulose 52柱分级法,用水、0.05、0.1、0.2和0.5mol/L NaCl盐溶液洗脱竹笋多糖;最后,通过Sephadex-50分级用水洗脱主要成分。 ZMP(一种多糖)的分离纯化[4] 从木枣果实中提取粗多糖,命名为ZMP,通过乙醇沉淀、冷冻干燥,然后用Sevag试剂脱蛋白。分离过程中ZMP的总收率为4.31%(图1),与先前研究中报道的结果相似。为了提高纯度并获得均匀的多糖产品,使用DEAE纤维素柱(2.6 cm×40 cm)的阴离子交换色谱法纯化ZMP。用去离子水(0.05、0.10和0.30 M NaCl)洗脱的四个组分分别命名为ZMP-1、ZMP-2、ZMP-3和ZMP-4(图2)。用去离子水洗脱的ZMP-1可能是中性多糖,而分别用0.05、0.10和0.30 mol/L NaCl溶液洗脱的ZMP-2、ZMP-3和ZMP-4是酸性多糖。使用蒸馏水作为洗脱剂,以1.0 mL/min的流速通过Sephadex G-100凝胶过滤色谱法进一步纯化四个组分中的每一个。自动收集洗脱液(8 mL/管),得到GZMP-1、GZMP-2、GZMP-3和GZMP-4(图3)。基于ZMP的原始量,四种馏分的回收率分别为0.209%、0.093%、0.418%和0.255%。所有四条洗脱曲线都有一个对称的单峰,这表明所有纯化产物都是均匀的多糖。 阴离子交换柱色谱法[6] 这是目前多糖纯化和柱层析中最常用的方法。特别是,阴离子交换柱色谱法通常首先用于体积较大的多糖溶液。通过这种方法,多糖溶液可以被浓缩和初步纯化,甚至一些多糖可以被纯化成均匀的组分。目前广泛使用的阴离子交换剂有DEAE纤维素、DEAE Sephadex和DEAE Sepharose,其中DEAE纤维素通常是首选。DEAE纤维素具有开放的骨架,多糖分子可以自由进入这种载体并迅速扩散。DEAE纤维素具有较大的表面积。尽管其离子交换容量仅为0.70⿿0.75 DEAE纤维素对多糖的吸附量远大于离子交换树脂。此外,由于纤维素上的离子交换基团较少、排列松散且呈碱性,DEAE纤维素对多糖的吸附较弱,多糖可以用一定离子浓度的盐溶液洗脱出来。 阴离子交换柱色谱法适用于分离各种酸性多糖、中性多糖和粘多糖。阴离子交换柱色谱的分离机理不仅是离子交换,还有吸附-解吸。因此,阴离子交换柱色谱法可用于中性和酸性多糖的分离,也可用于不同中性多糖的提取。一般来说,当pH值为6.0时,酸性多糖可以吸附到交换剂上,而中性多糖则不能吸附。然后,可以使用具有相同pH值和不同离子强度的缓冲液分别洗脱这些酸性多糖。多糖吸附交换剂的能力与多糖的结构有关。吸附能力通常随着多糖分子中酸性基团的增加而增加。对于线性分子,与较小分子量的多糖相比,较大分子量的中性多糖更容易被吸附。直链多糖的吸附能力大于支链多糖。在大多数情况下,100克DEAE纤维素可以负载0.5⿿1.5 g干多糖样品。洗脱模式通常是使用不同离子强度的缓冲液进行梯度洗脱或逐步洗脱。此外,中性多糖还可以与硼砂(四硼酸钠)形成配位化合物。基于此,有时将DEAE纤维素加工成硼砂型DEAE纤维素。当多糖溶液流过硼砂型DEAE纤维素柱时,多糖将与硼砂配位并吸附在柱上。然后用不同浓度的硼酸盐溶液洗脱柱。首先流出的洗脱液是与硼砂不配位的多糖组分,最后流出的洗脱物是与硼砂配位最强的多糖部分。 除了DEAE-纤维素外,其他两种阴离子交换剂,即DEAE-Sephadex和DEAE-Sepha rose也被广泛使用。DEAE-Sephadex系列有几种产品,如DEAE-Sehadex A25(通常用于分子量<30000的多糖纯化)和DEAE-Sefadex A50。DEAE-Sepharose系列也有几种产品,如DEAE-Sepha rose CL-6B(通常用于分子量>100000的多糖)。由于这些交换剂具有三维网络结构,它们不仅具有离子交换功能,还具有分子筛效应。与纤维素相比,它们具有更高的电荷密度,因此具有更大的交换容量和更好的分离效果。然而,当洗脱液的pH值或离子强度发生变化时,两种交换剂(DEAE Sephadex和DEAE Sepharose)的体积会发生很大变化,从而影响流速。DEAE Sephadex和DEAE Sepharose的再生工艺方法与DEAE纤维素相同。 综上所述,上述三种阴离子交换剂(DEAE纤维素、DEAE Sephadex和DEAE Sepharose)在多糖纯化中得到了广泛的应用。同时,也存在一些缺点,特别是当它们用于粘多糖纯化时。例如,流速低,柱床的高度可能会随着缓冲液浓度和pH值的变化而变化,因此不稳定,换热器的使用寿命短等。1990年后,这三种交换剂逐渐被另一种阴离子交换剂所取代,该阴离子交换剂的骨架是Sepharose FF,具有良好的化学稳定性和快速的流速⿿s.典型的产品型号为DEAE-Sepharose FF。DEAE-Seharose FF的使用方法类似于DEAE-纤维素。DEAE Sepharose FF不能以干粉形式储存,必须悬浮在水中保存。 DEAE纤维素的用量对金回收效率的影响[1] 第一个实验系列是通过将DEAE纤维素的量从10毫克改变到400毫克,同时保持其他参数不变来进行的。图2显示了金回收效率随DEAE纤维素量的增加而变化。从图中可以明显看出,回收效率随着DEAE纤维素量的增加而增加。 随着DEAE纤维素量的增加,金回收效率的提高可以很容易地解释为,吸附剂与待回收金属的高比例在热力学上有利于回收反应(Matsubara等人,2000)。 当氯化金溶液与DEAE纤维素接触时,三价(Au3+)形式的金被还原为金属形式(Auo),而DEAE纤维素中的羟基被氧化(Ogata和Nakano,2005)。XRD分析证明了这一点。净反应发生如下: DEAE纤维素中羟基的氧化表示如下 总体反应表示如下 当反应持续30分钟时,当DEAE纤维素的量为50毫克及以上时,回收率达到90%以上,超过100毫克后,回收率开始稳定在约100%。因此,选择10-60毫克的DEAE纤维素进行进一步的实验。 产品特性[1] 为了确定回收的金是否为金属形式,在800°C下烧掉DEAE纤维素后,对样品进行了XRD分析,然后获得了纯金。通过灰吹法测得金的纯度为99.8%。这表明金可以很容易地从DEAE纤维素中分离出来,得到纯金粉。在衍射图案中,在2θ=38、44.4、64.6、77.6、81.5处清楚地观察到峰,这与金属金峰一致。峰清楚地证实了溶液中存在的三价金离子被还原为金属金(Nakajima等人,2003,Ogata和Nakano,2005)。图8a和b显示了在800°C下燃烧DEAE纤维素前后回收的金颗粒的SEM图像。SEM图像显示,金原子聚集并沉积在DEAE纤维素的某些区域。在800°C下燃烧后,获得了多孔结构。 本研究表明,使用DEAE纤维素回收金对于从稀释的氯化金溶液中回收金是有效的。 使用过量的DEAE纤维素(DEAE纤维素/Au重量比为400及以上),即使在室温下,在30分钟后以130转/分的速度也很容易达到99%以上的金回收效率,而在相同条件下,将温度从30°C提高到60°C,可以使效率超过99%,同时DEAE纤维素的量显著减少(DEAE纤维/Au重量比约为120)。通过优化工艺参数,在相对较小的DEAE纤维素添加量下,金回收率接近理论最大值应该是可行的。根据已发表的文献,温度升高导致金回收效率提高。 发现金回收是通过将溶液中存在的三价金离子还原为金属金来实现的,SEM图像和XRD图案证明了这一点。 还表明,增加摇动速率和接触时间会提高金回收效率。考虑到10 mgDEAE纤维素的量,通过将振荡速率从20 rpm增加到120 rpm,金回收效率提高了约50%,这表明从稀释的含金溶液中回收金需要振荡。在本研究调查的所有参数中,发现摇动速率在回收效率方面最有效。根据已发表的文献,通过DEAE纤维素回收金是一个中间控制过程,活化能为37.11 kJ/mol,遵循一级动力学。 本研究表明,使用DEAE纤维素作为工业金回收中使用的传统吸附剂的替代品,可以成功实现金回收。此外,DEAE纤维素在金回收方面的突出特性为其他贵金属的有效回收提供了可能性 [1]。 |
| 酶活实验 |
使用DEAE纤维素从稀金溶液中回收金。[1]
该研究是在分批系统中通过一次改变一个回收参数进行的。对于每个实验,将10mL合成制备的50ppm金溶液与猎鹰管中的DEAE纤维素接触,以避免暴露在空气中。通过这种方式,空气无法扩散到系统中。实验在温控振荡水浴中进行,以确保猎鹰管表面均匀的热对流。 第一个实验系列是通过改变DEAE纤维素的量,同时保持其他参数不变来进行的。在第二个实验系列中,研究了在20rpm至140rpm范围内振动速率的影响。在温度受控的摇动水浴中以手动调节的摇动速率进行摇动。第三个实验系列研究了将反应时间从30分钟改变到120分钟的效果。在最后一个实验系列中,研究了温度在30-60°C范围内的影响。 每次运行后进行固体/液体分离。对于ICP分析,在适当稀释后将10mL过滤溶液引入机器。反应时间和溶液pH分别保持恒定在30分钟和4.07。在优化了DEAE纤维素的用量后,将其保持在10mg不变,用于动力学研究。因此,选择DEAE纤维素与金的重量比(10/0.5)约为20。 该回收过程的效率是使用以下方程式根据金回收率百分比计算的: 其中Co是初始金浓度(50ppm),Ct是实验结束时的浓度[1]。 |
| 毒性/毒理 (Toxicokinetics/TK) |
Other Multiple Dose Data oral/rat lowest published toxic dose: 159 gm/kg/90D- intermittent Nutritional and Gross Metabolic: Weight loss or decreased weight gain June 2017
Acute Toxicity Data oral/rat lowest published toxic dose: 120 gm/kg Gastrointestinal: Hypermotility, diarrhea; Gastrointestinal: Other changes June 2017 Acute Toxicity Data skin/rabbit lethal dose (50 percent kill): >2 gm/kg June 2017 Other Multiple Dose Data oral/rat lowest published toxic dose: 56000 mg/kg/8W- intermittent Blood: Thrombocytopenia; Blood: Other changes; Blood: Changes in other cell count (unspecified) June 2017 Acute Toxicity Data inhalation/rat lethal concentration (50 percent kill): >5800 mg/m3/4H June 2017 LC50 (rat) > 5,800 mg/m3/4h |
| 参考文献 |
|
| 其他信息 |
Cellulose is an odorless, white powdery fibers. Density: 1.5 g/cm3. The biopolymer composing the cell wall of vegetable tissues. Prepared by treating cotton with an organic solvent to de-wax it and removing pectic acids by extration with a solution of sodium hydroxide. The principal fiber composing the cell wall of vegetable tissues (wood, cotton, flax, grass, etc.). Technical uses depend on the strength and flexibility of its fibers. Insoluble in water. Soluble with chemical degradation in sulfuric acid, and in concentrated solutions of zinc chloride. Soluble in aqueous solutions of cupric ammonium hydroxide (Cu(NH3)4(OH)2).
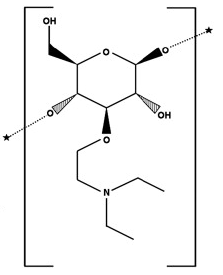
DEAE-cellulose is a glycoside. DEAE-cellulose has been reported in Aronia melanocarpa and Hyphaene thebaica with data available. A polysaccharide with glucose units linked as in CELLOBIOSE. It is the chief constituent of plant fibers, cotton being the purest natural form of the substance. As a raw material, it forms the basis for many derivatives used in chromatography, ion exchange materials, explosives manufacturing, and pharmaceutical preparations. The present work investigates gold recovery using DEAE-cellulose, a common biopolymer derivative, from synthetically prepared diluted gold-bearing solutions of 50 ppm. The effects of different recovery parameters on gold recovery efficiency were studied in detail. It was demonstrated that gold recovery efficiency increased with an increasing amount of sorbent, as well as increasing contact time. A gold recovery efficiency of 99% was attained under conditions of 20–40 g DEAE-cellulose per liter at a shaking rate of 130 rpm for 30 min at room temperature. On the other hand, with smaller amounts of sorbent (6 g/l), it was also possible to recover gold from the solution with 99% efficiency when the reaction temperature was increased to 60 °C. The shaking rate and temperature were demonstrated to play a vital role in the recovery process. It was also found that gold recovery by DEAE-cellulose is an intermediate-controlled process with an activation energy of 37.11 kJ/mol. The XRD pattern and SEM images revealed that the recovered gold was in the metallic form.[1] Native cellulose has a poor adsorption capacity and low physical stability because attachment of all three hydroxyls to the same ring may cause streric hindrance, and also the hydroxyl groups are not easily accessible to chemical reactions due to the crystalline regions in the polymer matrix (O'O et al., 2008, Mark et al., 1967). Modification by chemical reactions such as etherification, esterification, halogenation, and oxidation can develop adsorption capacity and structural stability of native cellulose for heavy metal ions (O'Connell et al., 2008). When the cellulose beads are mainly treated with 2-(diethylamino) ethyl chloride hydrochloride solution, and other procedures are carried out, DEAE-cellulose can be obtained (Ishimura et al., 1998). The molecular structure of DEAE-cellulose is shown in Fig. 1b. DEAE-cellulose is one of the most commonly used resins for ion-exchange chromatography containing an ionizable tertiary amine group and having less hydroxyls than native cellulose. The counterion of the DEAE-cellulose is Cl−. In this work, DEAE-cellulose, which is a weakly basic cellulose anion exchanger (Matsubara et al., 2000), was employed to recover gold from dilute gold chloride solutions. The objective of the present study was to outline an effective recovery process using DEAE-cellulose – a biopolymer derivative – as a sorbent for the high-efficiency recovery of gold from diluted gold-bearing solutions, and to describe the optimum conditions and parameters for this recovery process. For this purpose, the following parameters were studied to investigate their effect on gold recovery efficiency: DEAE-cellulose amount, reaction time, shaking rate, and temperature. Furthermore, the activation energy of the recovery process was calculated in a kinetic study. This study optimized the extraction process of crude polysaccharides from Panax notoginseng leaves (PNLP) using the ultrasonic-assisted dual-enzyme method through a single-factor combined with response surface experiment. The crude polysaccharides were subsequently purified and isolated with DEAE-cellulose 52, followed by structural analysis, evaluation of antioxidant activity, and examination of digestive enzyme inhibition. The hypoglycemic effects of the purified components were further clarified. The results indicated that the optimized crude polysaccharide had an extraction yield of 17.13 ± 0.29%. The purified fraction PNLP-3 (eluted with 0.3 M NaCl) was obtained through DEAE-Cellulose 52 chromatography, exhibiting a total sugar content of 81.2% and a molecular weight of 16.57 kDa. PNLP is primarily composed of arabinose, galactose, and galacturonic acid, with molar percentages of 20.24%, 33.54%, and 24.27%, respectively. PNLP-3 is mainly composed of arabinose and galactose, with molar percentages of 29.97% and 49.35%, respectively. In this study of hypoglycemic activity, the IC50 values of PNLP-3 for α-glucosidase and α-amylase inhibition were 1.045 mg/mL and 9.53 mg/mL, respectively. Molecular docking results confirmed that PNLP-3 exhibits better inhibitory activity against α-glucosidase. Furthermore, PNLP-3 alleviated hyperglycemia in insulin-resistant HepG2 cells by enhancing glucose consumption and glycogen synthesis. The antioxidant activity of PNLP-3 exhibited a positive correlation with its concentration, potentially contributing to its hypoglycemic effects by reducing oxidative stress. These findings underscore the therapeutic potential of Panax notoginseng leaf polysaccharides in managing type 2 diabetes and offer new perspectives on the use of natural polysaccharides for regulating blood glucose. [2] Three kinds of polysaccharides, namely, BSP1A, BSP2A, and BSP3B, were isolated from raw bamboo shoot (Dendrocalamus latiflorus) after purification and classification by DEAE-cellulose-52 (ion-exchange chromatography) and Sephadex G-50. The molecular weights of BSP1A, BSP2A, and BSP3B were 10.2, 17.0 and 20.0 kDa, respectively, which were measured through GPC (gel performance chromtatography) methods. BSP1A contained arabinose, glucose, and galactose in a molar ratio of 1.0:40.6:8.7. BSP2A and BSP3B contained arabinose, xylose, glucose, and galactose in molar ratios of 6.6:1.0:5.2:10.4 and 8.5:1.0:5.1:11.1, respectively. The existence of the O-glycopeptide bond in BSP1A, BSP2A, and BSP3B was demonstrated by β-elimination reaction. FTIR spectra of the three polysaccharides showed that both BSP2A and BSP3B contained β-d-pyranose sugar rings. However, BSP1A exhibited both β-d-pyranose and α-d-pyranose sugar rings. Congo red test indicated that BSP1A and BSP2A displayed triple helix structures, but BSP3B did not. NMR spectroscopy revealed that BSP1A may exhibit a β-1,6-Glucan pyran type as the main link, and few 1,6-glycosidic galactose pyranose and arabinose bonds were connected; BSP2A mainly demonstrated →5)β-Ara(1→and→3)β-Gal(1→connection. Furthermore, BSP3B mainly presented →3)β-Glu(1→and→3)β-Gal(1→connection and may also contain few other glycosidic bonds.[3] In the present study, crude polysaccharides from Ziziphus Jujuba cv. Muzao were isolated and purified using DEAE-cellulose-52 and Sephadex G-100 size-exclusion chromatography; four fractions were collected, namely GZMP-1, GZMP-2, GZMP-3, and GZMP-4. The molecular weights of these four fractions were measured to be 111.2, 95.1, 84.2, and 571.4 kDa, respectively, using high-performance gel permeation chromatography. Gas chromatography analysis of the monosaccharide composition confirmed that GZMP-1 was composed of rhamnose, arabinose, glucose, and galactose. Rhamnose, arabinose, and galactose were the main components present in GZMP-2 and GZMP-3, whereas GZMP-4 was composed of only rhamnose and arabinose. Scanning electron microscopy showed relatively smooth surfaces for GZMP-1 and GZMP-4, whereas GZMP-2 and GZMP-3 had more folds on their surfaces. Fourier transform infrared spectroscopy analyses indicated that GZMP-1 and ZMP mainly had α-type glycosidic linkages. The in vitro antioxidant activities of the polysaccharides revealed that jujube polysaccharides exhibit remarkable antioxidant activity, and can scavenge DPPH radical and OH radical in a concentration-dependent manner. The results of this work suggest that polysaccharides from Z. Jujuba cv. Muzao have potential to be used as functional food and in the development of natural antioxidant drug carriers. [4] The aim of this review is to point out the attention of the reader to the use of DEAE-cellulose/DEAE-C in organic reactions, possibly not only devoted to the preparation of heterocycles but potentially extending to other classes of organic compounds. Being DEAE-cellulose/DEAE-C an ammonium salt commonly used in chromatographic applications, it can be considered as a potential mild acid catalyst or a proton donor and these features can in theory catalyze standard acid-catalyzed organic reactions. In addition, the resin nature of DEAE-cellulose/DEAE-C could suggest the way to perform organic reactions in the solid state. [5] Polysaccharides play multiple roles and have extensive bioactivities in life process and an immense potential in healthcare, food and cosmetic industries, due to their therapeutic effects and relatively low toxicity. This review describes their major functions involved in antitumor, anti-virus, and anti-inflammatory bioactivities. Due to their enormous structural heterogeneity, the approaches for isolation and purification of polysaccharides are distinct from that of the other macromolecules such as proteins, etc. Yet, to achieve the homogeneity is the initial step for studies of polysaccharide structure, pharmacology, and its structure-activity relationships. According to the experiences accumulated by our lab and the published literatures, this review also introduces the methods widely used in isolation and purification of polysaccharides.[6] |
| CAS号 |
9013-34-7
|
|---|---|
| PubChem CID |
16211032
|
| 外观&性状 |
Solid powder
|
| 密度 |
1.1±0.1 g/cm3
|
| 沸点 |
519.1±50.0 °C at 760 mmHg
|
| 闪点 |
267.7±30.1 °C
|
| 蒸汽压 |
0.0±3.1 mmHg at 25°C
|
| 折射率 |
1.518
|
| LogP |
0.68
|
| tPSA |
94.86
|
| 别名 |
DEAE-CELLULOSE; Cellulose DEAE; Diethylaminoethyl-cellulose; DEAE-Sephacel(R); Diethylaminoethyl-Sephacel(R); (6S)-2-(hydroxymethyl)-6-[(3S)-4,5,6-trihydroxy-2-(hydroxymethyl)oxan-3-yl]oxyoxane-3,4,5-triol; (5S)-6-(hydroxymethyl)-5-{[(2S)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}oxane-2,3,4-triol; ...; 9013-34-7;
|
| HS Tariff Code |
2934.99.9001
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
1、使用方法
1.1色谱柱装填
( 1)让所有的材料和试剂均平衡至层析实验的温度。配制缓冲液,对所有的缓冲液进行脱气处理。
( 2)称取适量的填料,用纯水室温溶涨 4 小时,或用热水溶胀 1 小时(不要水浴)。溶胀好之后,用纯水清洗 5 倍柱体积。
( 3)检查层析柱所有部件,特别是过滤网是否完好,密封圈、螺旋塞是否紧密,玻璃管是否干净和完整。
( 4)将柱子底端用水或缓冲液润湿并保持一小段液位,务必使底端无气泡。
( 5)用玻璃棒引导匀浆沿着柱内壁一次性倒入柱内,注意勿使产生气泡。打开柱子出液口,使凝胶在柱内自由沉降,连结好柱子顶端柱头。
( 6)打开蠕动泵,让缓冲液用使用时流速的 1.33 倍的流速流过,使柱床稳定(注意压力不要超过填料最大耐压)。
1.2平衡柱子
用上样的平衡缓冲液平衡柱子后即可上样 。( 当流出液的 pH 和电导值与起始缓冲液相同时层析柱即完全平衡)。
1.3上样
样品的盐浓度和 pH 要尽量和平衡柱子的缓冲液一致,盐浓度过高或者 pH 过低也许挂不上 。通过透析或脱盐的方法进行缓冲液置换,将样品缓冲液转移至起始平衡缓冲液。
最常见的程序是让目标分子结合到离子交换柱上,其他杂质流出。然而,在一些情况下,离子交换柱结合杂质而使目标分子流出,这样的操作也是可以的。
缓冲液的离子强度应保持较低,以免干扰样品结合,推荐的操作 pH 应在缓冲液 pKa 的 0.5 个单位内,并且和目标分子的等电点( pI)相差至少一个 pH 单位 。
1.4蛋白的洗脱
对于 DEAE 纤维素填料,一般使用盐浓度递增或 pH 值递减(线性或者阶梯梯度)的方式来进行洗脱。
1.5再生
根据样品的性质,通常通过用高离子强度洗脱缓冲液,如 2M NaCl 对柱子进行洗涤,或改变缓冲液 pH,然后在平衡缓冲液中重新平衡来进行再生 。如 果填料吸附性能发生改变,诸如变性蛋白质或脂质的物质在再生过程中洗脱不下来,则需要通过在位清洗程序 CIP 来清除。
1.6在位清洗(CIP)
使用几次后,如果发现结合能力有明显改变,这时候需要进行在位清洗。通过用2-5倍柱床体积
的 0.1M NaOH 溶液在位清洗填料,随后立即用大量纯水彻底清洗直至中性,从而除去沉淀的蛋白质、疏水结合的蛋白质和脂蛋白。
2、保存
未使用的填料,4-25℃密闭保存。使用完的填料,用纯水彻底冲洗,保存在20%乙醇中,4℃保存。
3、注意事项
( 1)上样之前,样品必须经过膜过滤及去除色素,否则杂质及色素会被吸附到填料上,影响填料的正常使用 。 所有的缓冲液必须用 0.45um 的滤膜过滤 。
( 2)此填料颗粒比较细,所以一定要注意柱子要选择合适的筛网,以免漏出填料。
( 3)在使用过程中,避免使用高浓度的强酸强碱,酸和碱的浓度应低于 0.15M,碱会使流速变慢。
( 4)离子交换介质在选择层析柱时,避免使用细长柱,会增加实验操作压力。
( 5)不同的样品,吸附和洗脱方法不相同,可以根据相关的文献进行。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。