| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| Other Sizes |
|
| 靶点 |
Fluorescent dye
|
|---|---|
| 体外研究 (In Vitro) |
体外稳定性研究[1]
通过测量信号强度的变化,对分散在玉米油中的Cy-PP微塑料的荧光稳定性进行了10分钟的评估,以确认Cy-PP微型塑料的包封(图S4)。所有三个样品(Cy5.5-COOH、Cy-PP(约5µm)和Cy-PP的荧光强度在10分钟的暴露时间内保持在95%以上。此外,在体内生物分布研究之前,进行了体外消化过程模拟,以证明Cy-PP微塑料在体内的稳定性。模拟消化由三种溶液组成:唾液、胃液和肠液(分别为SF、GF和IF)。Cy-PP微塑料按照已知的方法经历了模拟消化系统的三个阶段的消化过程(图5a)。根据一系列消化步骤,没有观察到Cy5.5-COOH的显著释放,每种消化液的荧光信号很小(图5b)。 |
| 体内研究 (In Vivo) |
目前,聚丙烯(PP)用于各种产品中,因此导致人体每天接触量很高。因此,有必要评估PP微塑料在人体内的毒理学效应、生物分布和积累。在这项研究中,与ICR小鼠的对照组相比,施用两种粒径的PP微塑料(约5和10-50µm)不会导致包括体重和病理检查在内的几个毒理学评估参数发生任何显著变化。因此,确定了PP微塑料在ICR小鼠中的近似致死剂量和未观察到的不良反应水平为≥2000mg/kg。此外,我们制造了花青5.5羧酸(Cy5.5-COOH)标记的碎片化PP微塑料,以实时监测体内生物分布。小鼠口服Cy5.5-COOH标记的微塑料后,在胃肠道中检测到大多数PP微塑料,并在IVIS Spectrum CT中观察到24小时后排出体外。因此,本研究为PP微塑料在哺乳动物中的短期毒性、分布和积累提供了新的见解[1]。
|
| 酶活实验 |
聚丙烯微塑料的荧光标记[1]
使用CSD方法对PP微塑料进行荧光标记,以进行生物分布分析。首先,将15克PP微塑料加入150毫升蒸馏水和150毫升THF中,搅拌10分钟。将0.5毫升DMSO中的Cy5.5-COOH溶液(50毫克毫升-1)加入PP微塑料悬浮液中,搅拌4天。反应后,使用定性滤纸(2至3µm)通过真空过滤分离Cy-PP微塑料,以去除反应悬浮液中未标记的Cy5.5-COOH,然后用蒸馏水和乙醇洗涤。Cy-PP微塑料在40°C的暗处干燥。分别使用SEM和FT-IR光谱分析了Cy5.5标记的PP的形态和化学结构。 |
| 动物实验 |
Single Toxicity Test [1]
A single oral administration toxicity test was performed to observe the toxic reactions of two particle sizes of PP microplastics with a single oral administration and to confirm the ALD. Twelve male and female 6-week-old ICR mice were separated into the control, low-dose (500 mg/kg), medium-dose (1000 mg/kg), and high-dose (2000 mg/kg) groups for two different particle sizes of PP microplastics. Corn oil was administered to the control group, and PP microplastic suspended in corn oil at a dosage of 10 mL/kg liquid amount were administered to the other groups as a single oral administration. During the 2-week period, clinical sign observation (once a day), morbidity or dead animal observation (twice a day), and body weight measurement (once a week) were performed. After the end of the observation period, all animals were euthanized using carbon dioxide anesthesia and exsanguinated via the abdominal aorta, followed by necropsy and gross postmortem examination. All conditions for the experiment were set with reference to the OECD Test Guidelines 423 [60]. Four-Week Repeated Toxicity Test [1] A 4-week repeated oral administration toxicity test was conducted to evaluate the toxicity response and safety of PP microplastics. Forty male and female 6-week-old ICR mice were separated into the control, low-dose (500 mg/kg), medium-dose (1000 mg/kg), and high-dose (2000 mg/kg) groups. PP microplastic suspension in corn oil at a dosage of 10 mL/kg was orally administered to all the groups except the control group once a day for 4 weeks. During the 4-week observation period, clinical sign and morbidity or dead animal observations were performed once and twice a day, respectively. Body weight and food and water consumption were measured once a week. After the end of the observation period, blood was collected via the abdominal aorta under isoflurane anesthesia. A blood cell analyzer and a serum biochemistry analyzer were used to perform hematological and hematochemical analyses, respectively. Complete gross postmortem examinations were performed on all animals and tissues. The adrenal gland, brain, cecum, colon, duodenum, epididymis, esophagus, heart, ileum, jejunum, kidney, liver, lungs, ovary, pancreas, parathyroid gland, pituitary gland, rectum, spinal cord, spleen, stomach, testis, thymus, thyroid gland, trachea, and uterus were harvested. Among the extracted organs, the brain, spleen, heart, kidney, liver, testis, epididymis, and ovary were weighed. All extracted organs were fixed to 10% neutral-buffered formalin. For histopathological evaluation, a tissue processor was used for procedure of the tissues from the formalin-fixed samples. The paraffin-embedded tissue blocks were cut to a 4 µm thickness and mounted onto glass slides. Staining was performed with hematoxylin and eosin using an autostainer. The histopathological evaluation of all the slides was performed in a blind manner. In Vivo Biodistribution Study of Cy-PP Microplastics in Mice [1] Fifteen male and female 6-week-old ICR mice were separated into the control, Cy-PP (approximately 5 µm), and Cy-PP (10–50 µm) groups. ICR mice had their hair removed to minimize autofluorescence generated from the hair and were fasted 8 h before fluorescence imaging. Two particle sizes of Cy-PPs were orally administered to mice as a solution dispersed in corn oil at a concentration of 2000 mg/kg. Fluorescent images were acquired by the IVIS Spectrum CT at excitation and emission wavelength of 675 and 720 nm, respectively. In vivo images were acquired under 2.5% isoflurane anesthesia, and imaging process was performed at 0.2, 0.5, 1, 2, 4, 6, 8, and 24 h after administration. Twenty-four hours after oral administration of corn oil for control, Cy-PP (approximately 5 µm), and Cy-PP (10–50 µm), feces were collected from mice in group cages, and in vitro evaluation was performed. At 24 h after administration, in vivo imaging was the end point, saline perfusion was performed via the left ventricle of the mouse before sacrifice, and ex vivo imaging was performed. |
| 参考文献 | |
| 其他信息 |
We labeled the two particle sizes of PP microplastics with fluorescent dye Cy5.5-COOH. After oral administration of the labeled microplastics to the mice, most of labeled microplastics were observed in the gastrointestinal tract. Moreover, most of labeled microplastics were observed to be out of the body after 24 h in IVIS Spectrum CT. We observed that in the extracted organs, some microplastics partially remained in the gastrointestinal tract and were not observed in other organs. From the aforementioned results, it provides a new insight on the toxicological significance of two particle sizes of PP microplastics, along with the distribution and accumulation levels of PP microplastics in mammals. For future studies, long-term administration of microplastics with higher doses than the doses in this study and analysis of additional organs should be considered. [1]
|
| 分子式 |
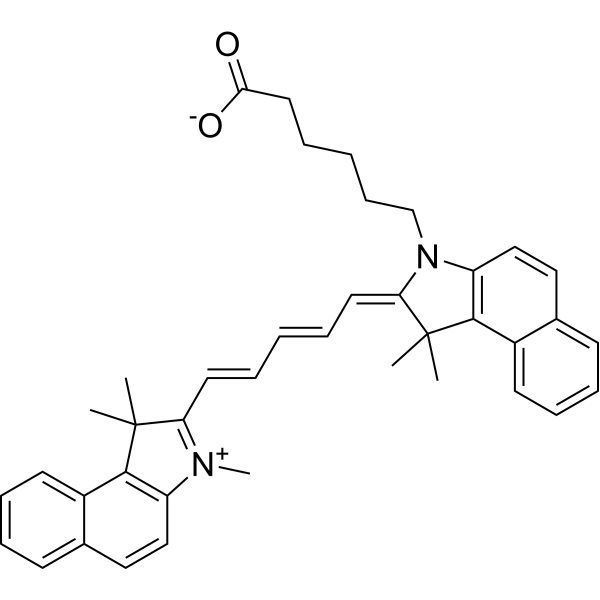
C40H42N2O2
|
|---|---|
| 分子量 |
582.773690700531
|
| 精确质量 |
582.324
|
| CAS号 |
1449612-07-0
|
| 相关CAS号 |
CY5.5-COOH chloride;2410537-32-3
|
| PubChem CID |
131637033
|
| 外观&性状 |
Brown to reddish brown solid powder
|
| LogP |
10
|
| tPSA |
46.4
|
| 氢键供体(HBD)数目 |
0
|
| 氢键受体(HBA)数目 |
3
|
| 可旋转键数目(RBC) |
8
|
| 重原子数目 |
44
|
| 分子复杂度/Complexity |
1170
|
| 定义原子立体中心数目 |
0
|
| SMILES |
CC1(C(N(CCCCCC(=O)[O-])C2C=CC3=CC=CC=C3C1=2)=CC=CC=CC1=[N+](C2C=CC3=CC=CC=C3C=2C1(C)C)C)C
|
| InChi Key |
MQFHWNVLHOOSOL-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C40H42N2O2/c1-39(2)34(41(5)32-25-23-28-16-11-13-18-30(28)37(32)39)20-8-6-9-21-35-40(3,4)38-31-19-14-12-17-29(31)24-26-33(38)42(35)27-15-7-10-22-36(43)44/h6,8-9,11-14,16-21,23-26H,7,10,15,22,27H2,1-5H3
|
| 化学名 |
6-[(2Z)-1,1-dimethyl-2-[(2E,4E)-5-(1,1,3-trimethylbenzo[e]indol-3-ium-2-yl)penta-2,4-dienylidene]benzo[e]indol-3-yl]hexanoate
|
| 别名 |
Cy5.5-cooh; 1449612-07-0; 6-(1,1-Dimethyl-2-(5-(1,1,3-trimethyl-1H-benzo[e]indol-3-ium-2-yl)penta-2,4-dien-1-ylidene)-1,2-dihydro-3H-benzo[e]indol-3-yl)hexanoate; 1144107-80-1; 6-[(2Z)-1,1-dimethyl-2-[(2E,4E)-5-(1,1,3-trimethylbenzo[e]indol-3-ium-2-yl)penta-2,4-dienylidene]benzo[e]indol-3-yl]hexanoate
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 本产品在运输和储存过程中需避光。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: 50 mg/mL (85.80 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 1.25 mg/mL (2.14 mM) (饱和度未知) in 10% DMSO + 40% PEG300 +5% Tween-80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 12.5 mg/mL澄清的DMSO储备液加入到400 μL PEG300中,混匀;再向上述溶液中加入50 μL Tween-80 +,混匀;然后加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.7159 mL | 8.5797 mL | 17.1594 mL | |
| 5 mM | 0.3432 mL | 1.7159 mL | 3.4319 mL | |
| 10 mM | 0.1716 mL | 0.8580 mL | 1.7159 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。