| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| Other Sizes |
|
| 靶点 |
EGFR[1]
|
|---|---|
| 体外研究 (In Vitro) |
本研究研究了参与阿氟替尼代谢的CYP同工酶,并评价了阿氟替尼及其代谢产物的酶抑制和诱导潜力。数据显示,人肝微粒体(HLMs)中的阿氟替尼主要由CYP3A4代谢,可催化形成AST5902。[1] < br >
考虑到AST5902< strong>的高暴露性以及阿氟替尼和AST5902的结构,我们还评估了AST5902的CYP3A4诱导电位。在低浓度下,AST5902抑制CYP3A4的mRNA转录,但其潜在机制尚不清楚。与阿氟替尼和利福平相比,AST5902的体外诱导作用不显著。然而,鉴于AST5902的血浆暴露,AST5902也可能导致cyp3a4敏感底物的临床DDI [1]。
|
| 体内研究 (In Vivo) |
在I/II期临床试验中,单剂量阿氟替尼的Cmax和AUC在20 - 240mg的剂量范围内以剂量依赖的方式升高。多次给药后,阿氟替尼暴露量的增加小于单次给药。AST5902 AUC在240mg剂量组显著升高,甚至超过阿氟替尼。此外,阿氟替尼在多次给药后显示出时间依赖性和剂量依赖性的清除率(CL/F)增加。CYP表型研究和CYP酶诱导表明,阿氟替尼是CYP3A4的底物和诱导剂。因此,阿氟替尼的自我诱导可能是临床试验中观察到的现象的原因。考虑到80mg剂量的人阿氟替尼暴露,推测当阿氟替尼与cyp3a4敏感底物(包括咪达唑仑和三唑仑)共给药时,可激活临床药代动力学ddi。考虑到激活妊娠X受体(PXR)可诱导CYP3A和CYP2C,需进一步评估阿氟替尼诱导CYP2C的潜力[1]。
|
| 酶活实验 |
阿氟替尼在HLMs中的代谢[1]
在开始实验之前,将HLMs在冰上轻轻解冻。然后,将3µM阿氟替尼(0.5 mg蛋白/mL)加入到100 mM磷酸盐缓冲盐水(PBS;pH 7.4)至总容积为100 μL。37℃孵育3min后,加入1.0 mM NADPH启动反应。孵育1小时后,用相同体积的冰凉乙腈混合终止反应。所有孵育重复进行,然后进行UPLC-UV/Q-TOF ms分析。 特异性CYP抑制剂对HLMs的影响[1] 采用HLMs研究CYP酶抑制剂对阿氟替尼代谢的影响。孵育混合物(100µL)由阿氟替尼(3µM)、HLMs (0.5 mg蛋白/mL)、NADPH (1 mM)、PBS (100 mM, pH 7.4)和选择性CYP抑制剂组成。化学抑制剂分别为:CYP1A/ 2c α-萘黄酮(2µM)、CYP2C8槲皮素(20µM)、CYP2C9磺胺苯唑(6µM)、CYP2B6/2C19噻氯匹定(24µM)、CYP2D6奎尼丁(8µM)、CYP2E1氯甲基唑(24µM)、CYP3A酮康唑(2µM)、ABT(1µM)。在加入底物之前,将这些抑制剂与HLMs在NADPH存在下预孵育10分钟。然后,在37°C下孵育60分钟开始反应。最后,加入100µL冰凉的乙腈终止反应。所有的孵育重复进行,并在没有或存在抑制剂的情况下评估代谢物的形成。 重组人CYP同工酶对阿氟替尼的代谢作用[1] 为了鉴定参与阿氟替尼代谢的特异性异构体,将3µM阿氟替尼与重组人CYP1A2、2A6、2B6、2C8、2C9、2C19、2D6、2E1、3A4或3A5 (25 pmol P450/mL)混合,总体积为100 μL。分别加入1 mM NADPH和100µL冰凉乙腈开始和终止反应。37℃孵育60 min。所有反应均重复进行,然后进行UPLC-UV/Q-TOF MS分析。 |
| 细胞实验 |
3 μM的阿氟替尼与人肝细胞37℃孵育3 h,初步鉴定代谢物为AST5902,占剩余阿氟替尼浓度的52%。其他代谢物占阿氟替尼的不到1.2%(未发表数据)。此外,AST5902发挥了CYP3A4的诱导潜能,这可能与阿氟替尼的诱导作用有关[1]。
阿氟替尼和AST5902对人CYP3A4酶的酶促作用[1] 为了评估酶的诱导作用,将7 × 105个肝细胞/mL接种于胶原包被的24孔板中,置于37℃加5% CO2的加湿培养箱中24 h。用人CYP3A4酶诱导剂利福平(10 μM)、阿氟替尼或AST5902(0.003、0.01、0.03、0.1、0.3、1、3或5 μM)或0.1% DMSO(对照组)处理肝细胞,每天1次,连续3天。处理后,按照制造商的方案用TRIzol进行RNA提取。采用PrimeScript RT试剂盒进行cDNA合成。采用SYBR green Premix Ex Taq试剂盒,在StepOnePlus实时PCR系统上进行实时PCR。CYP3A4的正向引物为5 ' -ATCACTAGCACATCATTTGGAG-3 ',反向引物为5 ' -GGAATGGAAAGGTTATTGAGAG-3 '。GAPDH正向引物为5′-AGAAGGCTGGGGCTCATTTG-3′,反向引物为5′-GAGGGGCCATCCACAGTCTTC-3′。以GAPDH为内标,采用比较阈值循环法定量cDNA水平。EC50为诱导剂在50%最大诱导效应时的浓度,由GraphPad Prism 5.0进行非线性回归测试得到。 |
| 参考文献 | |
| 其他信息 |
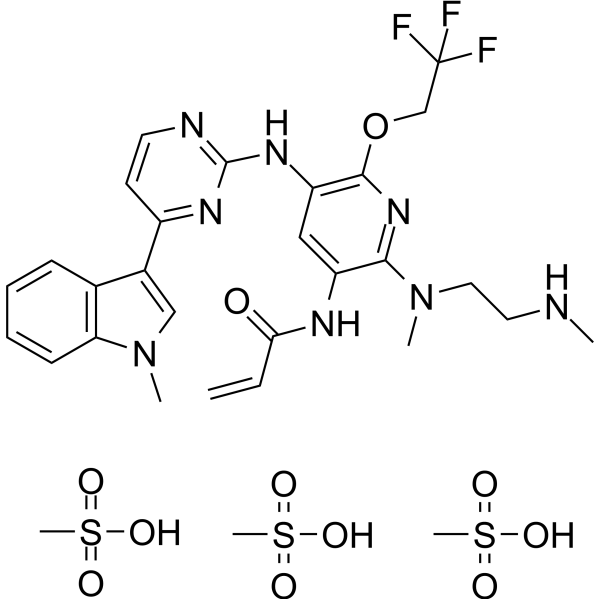
Alflutinib (AST2818) is a third-generation epidermal growth factor receptor (EGFR) inhibitor that inhibits both EGFR-sensitive mutations and T790M mutations. Previous study has shown that after multiple dosages, alflutinib exhibits nonlinear pharmacokinetics and displays a time- and dose-dependent increase in the apparent clearance, probably due to its self-induction of cytochrome P450 (CYP) enzyme. In this study, we investigated the CYP isozymes involved in the metabolism of alflutinib and evaluated the enzyme inhibition and induction potential of alflutinib and its metabolites. The data showed that alflutinib in human liver microsomes (HLMs) was metabolized mainly by CYP3A4, which could catalyze the formation of AST5902. Alflutinib did not inhibit CYP isozymes in HLMs but could induce CYP3A4 in human hepatocytes. Rifampin is a known strong CYP3A4 inducer and is recommended by the FDA as a positive control in the CYP3A4 induction assay. We found that the induction potential of alflutinib was comparable to that of rifampin. The Emax of CYP3A4 induction by alflutinib in three lots of human hepatocytes were 9.24-, 11.2-, and 10.4-fold, while the fold-induction of rifampin (10 μM) were 7.22-, 19.4- and 9.46-fold, respectively. The EC50 of alflutinib-induced CYP3A4 mRNA expression was 0.25 μM, which was similar to that of rifampin. In addition, AST5902 exhibited much weak CYP3A4 induction potential compared to alflutinib. Given the plasma exposure of alflutinib and AST5902, both are likely to affect the pharmacokinetics of CYP3A4 substrates. Considering that alflutinib is a CYP3A4 substrate and a potent CYP3A4 inducer, drug-drug interactions are expected during alflutinib treatment.[1]
Background Alflutinib is a novel irreversible and highly selective third-generation EGFR inhibitor currently being developed for the treatment of non-small cell lung cancer patients with activating EGFR mutations and EGFR T790M drug-resistant mutation. Alflutinib is mainly metabolized via CYP3A4 to form its active metabolite AST5902. Both alflutinib and AST5902 contribute to the in vivo pharmacological activity. The aim of this study was to investigate the effects of rifampicin (a strong CYP3A4 inducer) on the pharmacokinetics of alflutinib and AST5902 in healthy volunteers, thus providing important information for drug-drug interaction evaluation and guiding clinical usage. Methods This study was designed as a single-center, open-label, and single-sequence trial over two periods. The volunteers received a single dose of 80 mg alflutinib on Day 1/22 and continuous doses of 0.6 g rifampicin on Day 15-30. Blood sampling was conducted on Day 1-10 and Day 22-31. The pharmacokinetics of alflutinib, AST5902, and the total active ingredients (alflutinib and AST5902) with or without rifampicin co-administration were respectively analyzed. Results Co-administration with rifampicin led to 86% and 60% decreases in alflutinib AUC0-∞ and Cmax, respectively, as well as 17% decrease in AST5902 AUC0-∞ and 1.09-fold increase in AST5902 Cmax. The total active ingredients (alflutinib and AST5902) exhibited 62% and 39% decreases in AUC0-∞ and Cmax, respectively. Conclusions As a strong CYP3A4 inducer, rifampicin exerted significant effects on the pharmacokinetics of alflutinib and the total active ingredients (alflutinib and AST5902). The results suggested that concomitant strong CYP3A4 inducers should be avoided during alflutinib treatment. This trial was registered at http://www.chinadrugtrials.org.cn . The registration No. is CTR20191562, and the date of registration is 2019-09-12. Source: Invest New Drugs. 2021 Aug;39(4):1011-1018. doi: 10.1007/s10637-021-01071-z. |
| 分子式 |
C30H41F3N8O11S3
|
|---|---|
| 分子量 |
842.883753538132
|
| 精确质量 |
842.2
|
| CAS号 |
2929417-90-1
|
| 相关CAS号 |
AST5902;2412155-74-7
|
| PubChem CID |
155971210
|
| 外观&性状 |
Yellow to orange solid powder
|
| tPSA |
298Ų
|
| 氢键供体(HBD)数目 |
6
|
| 氢键受体(HBA)数目 |
20
|
| 可旋转键数目(RBC) |
11
|
| 重原子数目 |
55
|
| 分子复杂度/Complexity |
929
|
| 定义原子立体中心数目 |
0
|
| InChi Key |
KRCDPFVVSHJNOV-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C27H29F3N8O2.3CH4O3S/c1-5-23(39)33-20-14-21(25(40-16-27(28,29)30)36-24(20)37(3)13-12-31-2)35-26-32-11-10-19(34-26)18-15-38(4)22-9-7-6-8-17(18)22;3*1-5(2,3)4/h5-11,14-15,31H,1,12-13,16H2,2-4H3,(H,33,39)(H,32,34,35);3*1H3,(H,2,3,4)
|
| 化学名 |
methanesulfonic acid;N-[5-[[4-(1-methylindol-3-yl)pyrimidin-2-yl]amino]-2-[methyl-[2-(methylamino)ethyl]amino]-6-(2,2,2-trifluoroethoxy)pyridin-3-yl]prop-2-enamide
|
| 别名 |
AST5902 trimesylate; AST5902 (trimesylate); AST5902 trimesylate; 2929417-90-1; AST5902 (trimesylate);
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中(例如氮气保护),避免吸湿/受潮和光照。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: 50 mg/mL (59.32 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (2.97 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (2.97 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.1864 mL | 5.9320 mL | 11.8641 mL | |
| 5 mM | 0.2373 mL | 1.1864 mL | 2.3728 mL | |
| 10 mM | 0.1186 mL | 0.5932 mL | 1.1864 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。