| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 25g |
|
||
| Other Sizes |
|
| 药代性质 (ADME/PK) |
Metabolism / Metabolites
Butanediol is metabolized by the liver... beta-hydroxybutyric acid /a main metabolite/ is further metabolized in the tricarboxylic acid cycle to carbon dioxide, which accounts for about 90% of the dose administered. In other studies... in which rats were fed 1,3-butanediol for 3 to 7 weeks, it was found that the blood level of beta-hydroxybutyrate, was also higher than normal. R- and S-1,3-butylene glycol are taken up by the isolated liver of fed or starved rats at the same rate. R-1,3-butylene glycol is mainly transformed to the physiological ketone bodies R-3-hydroxybutyrate and acetoacetate. Only 29-38&% of the S-enantiomer are converted into physiological ketone bodies. The S-enantiomer is further metabolised to S-3-hydroxybutyrate (not a natural compound), lipids and carbon dioxide. Based on these results it can be concluded that the test item is metabolised via physiological pathways, suggesting that it has a low potential to accumulate. |
|---|---|
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION AND USE: 1,3-Butanediol is an odorless, colorless, viscous liquid with a sweet flavor and bitter aftertaste. It is used as an intermediate in manufacture of polyester plasticizers; humectant for cellophane, tobacco; and in the cosmetic and pharmaceutical industry as a glycerin substitute.1,3-Butanediol also has some mold inhibiting action. It is not registered for current pesticide use in the U.S., but approved pesticide uses may change periodically and so federal, state and local authorities must be consulted for currently approved uses. HUMAN EXPOSURE AND TOXICITY: 1,3-Butanediol is not irritating to human skin or mucous membranes. When applied to the human eye it causes immediate severe stinging, but irrigation with water brings rapid complete relief. ANIMAL STUDIES: Eye irritation occurred in one individual, although its irritant potency in rabbits appeared low. It was of low acute oral toxicity to rodents. No treatment-related effects on mortality, body weight gain, organ weights, hematology, histopathology or neoplastic changes were observed in rats fed 1,3-butanediol in the diet at 1, 3 or 10% (~ 643, 1960 or 6230 mg/kg-bw/day for males or ~ 844, 2330 or 7300 mg/kg-bw/day for females) for two years. Feeding studies in which 1,3-butylene glycol replaced carbohydrate as an energy source found central nervous system effects in rats, dogs and calves. 1,3-Butanediol decreased glucose and increased beta-hydroxybutyrate in lactating goats fed 1,3-butanediol in their diets.1,3-Butylene glycol was fetotoxic when fed to rats during pregnancy, and a multigeneration feeding study in rats gave some indication of reduced male fertility. No genotoxic activity (dominant lethality or chromosomal damage) was seen in rats treated orally. Interactions 1,3-Butanediol and phlorhizin were used to induce ketonemia and hypoglycemia in steers. Oral administration of butanediol increased blood beta-hydroxybutyrate (BHB) and plasma nonesterified fatty acids (NEFA) and decreased serum glucose. Subcutaneous injections of phlorhizin, given in addition to butanediol orally, further increased NEFA and BHB concentrations and decreased glucose. Dietary niacin supplementation of steers given phlorhizin and butanediol caused serum glucose concentration to increase and blood BHB and plasma NEFA concentrations to decrease. Evidence previously reported suggest that 1,3-butanediol (BD) enhances the hepatotoxic effect of a single small dose of carbon tetrachloride (CCl4) in a dose-related manner. The present study provides additional information concerning the quantitative relationship between the severity of the ketotic state produced by BD and the magnitude of the potentiation observed and emphasizes the use of ketone bodies (KB) to predict the potential hazard of the BD-CCl4 interaction. Liver damage was modulated in male Sprague-Dawley rats by varying the concentration of the BD solutions ingested prior to a CCl4 challenge (0.1 ml/kg, i.p.). These data were compared to ketone bodies in plasma, hepatic tissue and urine. BD produced a dose-dependent metabolic ketosis observable at dosages between 1.1 and 9.9 g/kg per day given for 7 days. Plasma and liver data correlated well together. Concomitantly, potentiation of the CCl4-induced liver injury was also dose-related for the same dosage range; the minimum effective dosage of BD for potentiation was estimated as 1.1 g/kg per day. The linear correlations between hepatic or plasma KB values and the indices of hepatic dysfunction (ALT, OCT) were highly significant. Using a semiquantitative method, a correlation was also found for the urinary KB data. These results suggest that plasma KB concentrations might be useful for predicting possible potentiation of the hepatonecrotic effect of CCl4 by BD. For 28 days, four steers received l,3-butanediol, which causes ketonemia, and phlorizin, which causes glucosuria. Steers also were fasted for 9 days. Effects of treatments on concentrations of metabolites in blood and liver and on kinetics of glucose metabolism were determined. Treatments were: control, control with dietary butanediol plus injected phlorizin, and fasting. Fasting caused hypoinsulinemia and decreased liver glycogen by 60%. Butanediol plus phlorizin and fasting caused 18% and 19% decreases of plasma glucose and 2.5- and 6-fold increses of free fatty acid concentrations in blood plasma. Glucose irreversible loss averaged 371, 541, and 182 g/day during control, butanediol plus phlorizin treatment, and fasting. Butanediol plus phlorizin increased-liver ketone body concentrations, caused glucosuria, ketonuria, and ketonemia, but did not affect insulin, glucagon, or growth hormone concentrations in plasma or triglyceride and glycogen contents in liver. Steers given butane plus phlorizin did not show all the usual signs of lactation ketosis, but the treatment still offers promise for studying causes and effects of ketosis. Rats maintained on 1,3-butanediol exhibit potentiated cholestatic responses to taurolithocholate or manganese-bilirubin injections; with alpha-naphthylisothiocyanate, the hyperbilirubinemia is enhanced but not the depression in bile flow. Non-Human Toxicity Values LD50 Rat oral 22800 mg/kg. LD50 Mice sc 16.5 mL/kg LD50 Rat sc 20.1 mL/kg LD50 Guinea pig oral 11 g/kg For more Non-Human Toxicity Values (Complete) data for 1,3-BUTANEDIOL (6 total), please visit the HSDB record page. |
| 参考文献 | |
| 其他信息 |
Butane-1,3-diol is a butanediol compound having two hydroxy groups in the 1- and 3-positions. It is a butanediol and a glycol.
1,3-Butanediol is found in pepper (c. annuum). 1,3-Butanediol is a solvent for flavouring agents 1,3-Butanediol is an organic chemical, an alcohol. It is commonly used as a solvent for food flavouring agents and is a co-monomer used in certain polyurethane and polyester resins. It is one of four stable isomers of butanediol. In biology, 1,3-butanediol is used as a hypoglycaemic agent. 1,3-Butanediol belongs to the family of Secondary Alcohols. These are compounds containing a secondary alcohol functional group, with the general structure HOC(R)(R') (R,R'=alkyl, aryl). See also: Avobenzone; butylene glycol (component of) ... View More ... Therapeutic Uses /EXP THER/ This study examined the effect of 1,3-butanediol on the selective loss of CA1 pyramidal neurons following a short period of near-complete forebrain ischemia. Injection of 55 mmol 1,3-butanediol/kg body weight at 24 h of recirculation and again at 36 hr following 10 min of forebrain ischemia markedly reduced damage to CA1 neurons examined at 72 hr of recirculation compared with that in saline-treated rats. Comparable treatment with ethanol did not cause significant protection. Neuronal loss was also not reduced by 1,3-butanediol treatment when the ischemic period was extended to 15 min or by single treatments at 24 hr or 36 hr following 10 min of ischemia. However, a single treatment 5 min after reversal of 10 min of ischemia was effective in ameliorating cell loss. The difference in effectiveness of 1,3-butanediol following 10 min and 15 min of ischemia is consistent with a number of previous studies, indicating that the processes leading to loss of CA1 neurons are modified when the ischemic period is extended. Previous findings that 1,3-butanediol reduced damage in other ischemia-susceptible neuronal subpopulations but not in CA1 neurons most likely reflected the longer period of ischemia which was used. The results of the present investigation demonstrate that administration of 1,3-butanediol offers a novel approach for interfering with post-ischemic loss of CA1 neurons following a brief ischemic period which is effective even when initiated after prolonged recirculation periods. /EXP THER/ The biochemical effect of S-1,3-butanediol on streptozotocin induced diabetic rats was studied. Rats were made diabetic by the intraperitoneal injection of 40 mg/kg body weight streptozotocin in sodium citrate buffer. A dosage of 25 mmol/kg body weight of S-1,3-butanediol was injected intraperitoneally for treatment. The streptozotocin induced diabetic rats showed a marked increase in blood glucose level, and significant increase in the level of cholesterol, triglycerides and free fatty acids. The glycogen levels in liver and kidney were greatly decreased in diabetic rats. Treatment with butanediol normalized the glucose and glycogen level but had no significant effect on protein and lipid levels. /EXP THER/ We previously showed that intrastriatal administration of aminooxyacetic acid (AOAA) produces striatal lesions by a secondary excitotoxic mechanism associated with impairment of oxidative phosphorylation. In the present study, we show that and the specific complex I inhibitor rotenone produces a similar neurochemical profile in the striatum, consistent with an effect of AOAA on energy metabolism. Lesions produced by AOAA were dose-dependently blocked by MK-801, with complete protection against GABA and substance P depletions at a dose of 3 mg/kg. AOAA lesions were significantly attenuated by pretreatment with either 1,3-butanediol or coenzyme Q10, two compounds which are thought to improve energy metabolism. These results provide further evidence that AOAA produces striatal excitotoxic lesions as a consequence of energy depletion and they suggest therapeutic strategies which may be useful in neurodegenerative diseases. /EXP THER/ In order to assess the therapeutic value of 1,3 butanediol in ethylene glycol toxicosis, mixed-bred dogs were given an oral dose of commercial antifreeze at 6 mL/kg of body weight (0 hour) and treated (IV) 7 times at 6-hour intervals with 5.5 mL/kg of body weight 1,3 butanediol solution (20% in physiological saline solution) beginning at 8, 12, and 21 hours. Serum glycolic acid concentration was quantitated by high-pressure liquid chromatography. Three dogs that were given ethylene glycol, but no 1,3 butanediol treatment, died with elevated serum glycolic acid concentrations. Five dogs were given ethylene glycol and 1,3 butanediol treatment. Of 2 dogs treated at 8 hours, 1 survived and 1 died at 39 hours; 1 treated at 12 hours and 1 treated at 21 hours survived; 1 dog died soon (27 hours) after treatment was initiated at 21 hours. Four of the 5 dogs had dramatically decreased serum glycolic acid concentrations after 1,3 butanediol treatment, indicating its effectiveness in inhibiting alcohol dehydrogenase-dependent glycolic acid formation in vivo. /EXP THER/ Pre-partum feeding of 1,3-butanediol to sows has been shown to improve the metabolic status and survival rate of neonatal pigs. To evaluate the efficacy of short-term, pre-partum feeding of 1,3-butanediol on pig and sow productivity on a large scale and low concentration was the focus of the research. The secondary objective was to determine if pre-partum feeding of 1,3-butanediol had any effect on survival rate and weight gain of lesser body weight pigs, sow body weight and subsequent sow reproductive performance. In a large commercial unit, 2537 sows were fed one of two pre-partum diets (0% or 4.55% 1,3-butanediol) on Day 108+/-3 of pregnancy. 1,3-butanediol provided 8% of the total metabolizable energy. Pigs born live in those litters were equalized by cross-fostering among sows receiving the same pre-partum diet. Pigs were weaned from the sows at 16+/-3 days post-partum and return of sows to estrus and conception rates were determined. Pre-partum feeding of 1,3-butanediol reduced (P=0.01) pre-weaning pig mortalities from 1.44 to 1.24 pigs per litter. The reduction in pig mortality was independent of length of 1,3-butanediol feeding (4 to 11 days). In a subset of 750 litters, four lesser birth-weight pigs from each litter were tagged and monitored to determine the effect of 1,3-butanediol on survival rates and pre-weaning weight gain of pigs with the greatest mortality risk. 1,3-butanediol reduced (P=0.01) pre-weaning mortality of these low birth weight pigs by 5.27%. Based on these data, short-term pre-partum feeding of 1,3-butanediol effectively improves pre-weaning pig productivity at a lower concentration than previously reported. |
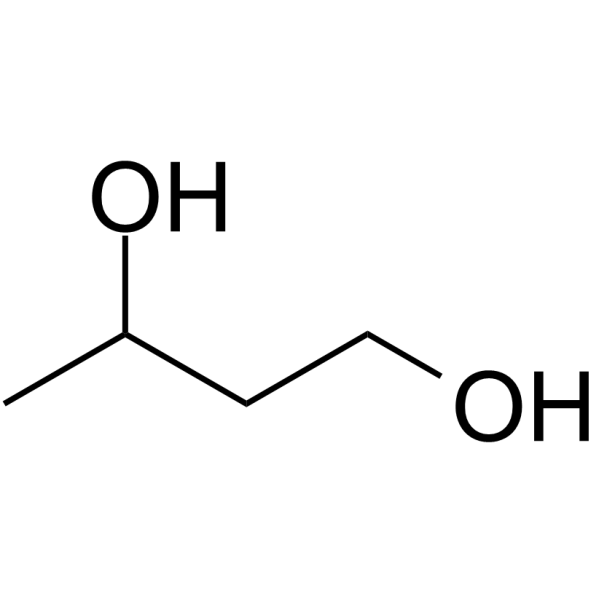
| 分子式 |
C4H10O2
|
|---|---|
| 分子量 |
90.12
|
| 精确质量 |
90.068
|
| CAS号 |
107-88-0
|
| 相关CAS号 |
(R)-(-)-1,3-Butanediol;6290-03-5
|
| PubChem CID |
7896
|
| 外观&性状 |
Viscous liquid
Pure compound is colorless |
| 密度 |
1.0±0.1 g/cm3
|
| 沸点 |
207.0±0.0 °C at 760 mmHg
|
| 熔点 |
-54ºC
|
| 闪点 |
121.1±0.0 °C
|
| 蒸汽压 |
0.1±0.8 mmHg at 25°C
|
| 折射率 |
1.438
|
| LogP |
-0.69
|
| tPSA |
40.46
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
2
|
| 可旋转键数目(RBC) |
2
|
| 重原子数目 |
6
|
| 分子复杂度/Complexity |
28.7
|
| 定义原子立体中心数目 |
0
|
| SMILES |
O([H])C([H])(C([H])([H])[H])C([H])([H])C([H])([H])O[H]
|
| InChi Key |
PUPZLCDOIYMWBV-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C4H10O2/c1-4(6)2-3-5/h4-6H,2-3H2,1H3
|
| 化学名 |
butane-1,3-diol
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
H2O: ≥ 500 mg/mL (5548.16 mM)
DMSO: 100 mg/mL (1109.63 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (27.74 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (27.74 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 悬浮液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL 澄清 DMSO 储备液加入到 900 μL 玉米油中并混合均匀。 View More
配方 3 中的溶解度: ≥ 1.72 mg/mL (19.09 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 11.0963 mL | 55.4816 mL | 110.9632 mL | |
| 5 mM | 2.2193 mL | 11.0963 mL | 22.1926 mL | |
| 10 mM | 1.1096 mL | 5.5482 mL | 11.0963 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。