| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| Other Sizes |
|
| 靶点 |
BCL6
|
|---|---|
| 体外研究 (In Vitro) |
含氯和氟取代吡啶的化合物CCT374705具有最佳的性质平衡,包括增加的溶解度和微粒体清除率,类似于CCT374284[1]。
|
| 体内研究 (In Vivo) |
我们选择使用Karpas 422异种移植物模型对CCT374705进行疗效研究,在该细胞系中CCT347405具有最强的抗增殖作用。本工作的目的是研究BCL6在体内的抑制作用。为了评估这一点,我们希望确保我们的抑制剂(>IC90)在整个研究中完全覆盖。为了达到这种高水平的持续暴露,该研究采用每天两次50 mg/kg的口服给药方案进行。我们能够通过测量ARID3A mRNA表达的增加来证实靶点与BCL6的结合。PK/PD分析显示CCT374705的游离浓度在给药后12小时内保持远高于游离IC90。然而,与我们之前报道的BCL6降解剂CCT373566的疗效结果类似,仅观察到中等的体内疗效。在优化了CCT374705的体内药代动力学特征后,该化合物是检测BCL6在小鼠模型内疾病中的作用和功能的合适探针[1]。
|
| 酶活实验 |
TR-FRET测定[1]
在384孔黑色Proxiplate(Perkin-Elmer)中在测定缓冲液(25mM Hepes pH8、100mM NaCl、0.05%Tween20、0.5mM TCEP、0.05%牛血清白蛋白)中进行测定,所述Proxiplated含有1nM Trx-6xHis-BCL6(内部生产的人BCL6 BTB结构域覆盖氨基酸序列5-129)、300nM BCOR-AF633肽(RSEIISTAPSSWVVPGP Cys-AlexaFluor 633酰胺)和0.5nM抗6xHis-铒隐窝。使用ECHO550声学分配器将DMSO或单独DMSO中的测试化合物添加到孔中,以在0.7%v/v DMSO中得到合适的测试浓度。在室温下孵育2小时后,在具有337nm激光激发、第一发射滤光片APC 665nm和第二发射滤光片Europium 615nm的Envision读板器上读取板,或者在配备有337nm激光激励滤光片、620nm的第一发射滤光片和665nm的第二发射滤光器的Pherastar FSX读板器中读取板。通过将FRET比率标准化为适当的高(DMSO具有所有试剂)和低(DMSO不具有BCL6)对照来计算在每个浓度下的抑制%。IC50值使用GraphPad Prism 6.0或Domatics软件通过将归一化数据拟合到S形四参数逻辑拟合方程来确定。 NanoBRT测定[1] 细胞纳米生物发光共振能量转移(nanoBRET)测定法用于检测BCL6-SMRT(也称为NCOR2)辅压蛋白-蛋白相互作用的抑制作用。将编码全长BCL6和SMRT的DNA插入pFC32K中。NanoLuc和pFC14K。HaloTag载体,分别产生C末端标记的融合蛋白BCL6 nanoLuc和SMRT HaloTag。将HEK293T细胞接种(5x105)在T75组织培养瓶中,48小时后用Fugene 6试剂和编码BCL6 nanoLuc作为供体和SMRT HaloTag作为受体的18µg总DNA质粒以1:25的供体与受体DNA比例进行批量转染。转染后24小时,收集HEK293T细胞,并将其储存在90%FBS和10%DMSO的液氮中。在测定时,使用Echo550声学分配在干燥的384孔NUNC白色测定板中分配化合物(100nL/孔)和NanoBRET 618配体(10nL的1mg/ml储备溶液/孔)。将冷冻转染的HEK293T细胞解冻、离心,并用不含酚红的OptiMEM+4%FBS代替冷冻培养基。将细胞密度调节至3x105个细胞/ml,并将20µL(6000个细胞)接种在每个孔中,每个孔含有试验化合物(0.0125-50µM)的DMSO或DMSO单独溶液和0.5µg/ml NanoBRET 618荧光配体,最终浓度为0.55%v/v DMSO。将细胞在37°C/5%CO2下孵育6小时,然后加入NanoBRET呋嗪底物,得到10µM的最终浓度。在短暂离心后,在配备有LUM/D600双镜、LUM 450/40nm带通滤波器和D605nm长通滤波器的Envision(Perkin-Elmer)读板器上读取板,读取0.2秒以确定BRET比。或者,在配备有BRET模块LP610nm(第一发射滤光片)/450-80nm(第二发射滤光片)的Pherastar FSX上读取板。通过将BRET比率标准化为适当的高和低对照,计算每个试验浓度下的抑制%。使用Graphpad Prism 6.0或Domatics软件通过将归一化数据拟合到S形四参数逻辑拟合方程来确定化合物IC50。 |
| 细胞实验 |
将细胞以2500个细胞/孔的密度接种在96孔培养板中,接种在补充有10%FBS的RPMI-1640培养基中。首先使用Echo 550声学分配器将化合物分配到96孔U形底板中,然后在RPMI-1640%培养基中稀释并转移到细胞上。用8种浓度的化合物处理细胞,一式两份,范围从1.07 nM到10µM,最终DMSO浓度为0.1%,最终体积为100µl。将细胞与化合物孵育14天,第3、7和10天的培养基变化如下:制备新鲜的96孔细胞培养板,其中含有100µl培养基加上测定浓度的化合物(第10天使用白色平板以优化发光测量)。将含有细胞的测定板涡旋混合,并使用Coulter Z2细胞计数器计数一个对照孔中的细胞密度。计算对照孔中含有2500个细胞的培养基的体积,并将该体积的细胞从测定板的每个孔转移到含有化合物的新鲜板的相应孔中。14天后,将CellTiter-Glo reagen以1:2的比例添加到测定板的每个孔中的培养基中,在摇板器上混合,然后在室温下孵育10分钟。使用Envision读板器测量发光,并计算在每种化合物浓度下与单独的DMSO相比的相对发光。GI50是使用Dotmatics中的4参数曲线拟合来确定的[1]。
|
| 动物实验 |
In vivo pharmacokinetic studies[1]
Animals were adapted to laboratory conditions for at least 1 week prior to dosing and were allowed food and water ad libitum. Compounds were administered iv or po (mouse, 0.1 mL/10 g in 10% DMSO, 5% tween 20 in saline); blood samples were collected in heparinised capillaries from the tail vein at 7 or 8 time points over 6 or 24 h post dose and frozen on collection together with a standard curve and quality controls spiked in control blood. Samples were reconstituted in a water:MeOH mixture containing internal standard as previously described (Roberts et al, 2016). Following centrifugation, extracts were analyzed by multiple reaction monitoring of precursor and product ions by ESI-LCMS/MS on either a Waters Xevo TQ-S or Sciex QTrap6500 following gradient separation with 0.1% formic acid and methanol on a Phenomenex Kinetex C18 UPLC column (50 × 2.1 mm, 2.6 μM). Quantitation was carried out with an external calibration. Quality controls were included and were within 20% of nominal concentration. Pharmacokinetic parameters were derived from noncompartmental analysis using Phoenix Pharsight Non compartmental analysis (model 200 and 201) version 6.3. All parameters are calculated from timepoints up to 6 h. Formulation of CCT374705[1] A solution formulation suitable for higher concentrations (> 5 mg/mL) of CCT374705 was developed by SEDA as described in Table S4. CCT374705 was dissolved in a pre-determined volume of DMSO and to this was added a pre-determined volume of Kolliphor HS15 at 40 °C. The solution was briefly vortexed before addition of a pre-determined volume of PEG400 at 40 °C. After a brief vortex a pre-determined volume of aqueous HPMC (1.25%, viscosity 40-60cp grade) was added. The formulation was vortexed and then sonicated at 40 oC for ~45- 60 mins. Complete vehicle was prepared in the same manner with no compound added. The formulation was stored at room temperature for 3-4 days maximum. Both compound and vehicle solutions were delivered orally (0.2 mL per 20 g mouse) using a gavage needle - mice were individually weighed and dosing volumes adjusted accordingly. In vivo pharmacodynamic (PK/PD) and efficacy studies [1] Preparation of Karpas 422 xenograft model in mouse. Karpas 422B cells were prepared for injection at a final concentration of 5 x107 cells/mL, using serum–free RMPI-1640 and an equal volume of Matrigel®, both chilled to 4°C. Cells were delivered to female SCID mice at 107 per 200uL, subcutaneously, single site. At three weeks post injection tumour bearing mice (mean diameter 0.5 cm2 +/- 0.1) were randomly selected and assigned to a treated group and a control group (n = 10 per group). CCT374705 and vehicle were formulated as described in Supplementary experimental 5.2: CCT374705 (50 mg/kg) and vehicle (0.2 mL/20g body weight) were administered orally, twice per day, approximately 12 hours apart for 35 days. |
| 参考文献 | |
| 其他信息 |
B-cell lymphoma 6 (BCL6) is a transcriptional repressor and oncogenic driver of diffuse large B-cell lymphoma (DLBCL). Here, we report the optimization of our previously reported tricyclic quinolinone series for the inhibition of BCL6. We sought to improve the cellular potency and in vivo exposure of the non-degrading isomer, CCT373567, of our recently published degrader, CCT373566. The major limitation of our inhibitors was their high topological polar surface areas (TPSA), leading to increased efflux ratios. Reducing the molecular weight allowed us to remove polarity and decrease TPSA without considerably reducing solubility. Careful optimization of these properties, as guided by pharmacokinetic studies, led to the discovery of CCT374705, a potent inhibitor of BCL6 with a good in vivo profile. Modest in vivo efficacy was achieved in a lymphoma xenograft mouse model after oral dosing.[1]
|
| 分子式 |
C21H18CLF3N4O2
|
|---|---|
| 分子量 |
450.841434001923
|
| 精确质量 |
450.10703
|
| CAS号 |
2640647-90-9
|
| PubChem CID |
156276733
|
| 外观&性状 |
Typically exists as white to off-white solids at room temperature
|
| LogP |
4.2
|
| tPSA |
66.5Ų
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
8
|
| 可旋转键数目(RBC) |
3
|
| 重原子数目 |
31
|
| 分子复杂度/Complexity |
765
|
| 定义原子立体中心数目 |
1
|
| SMILES |
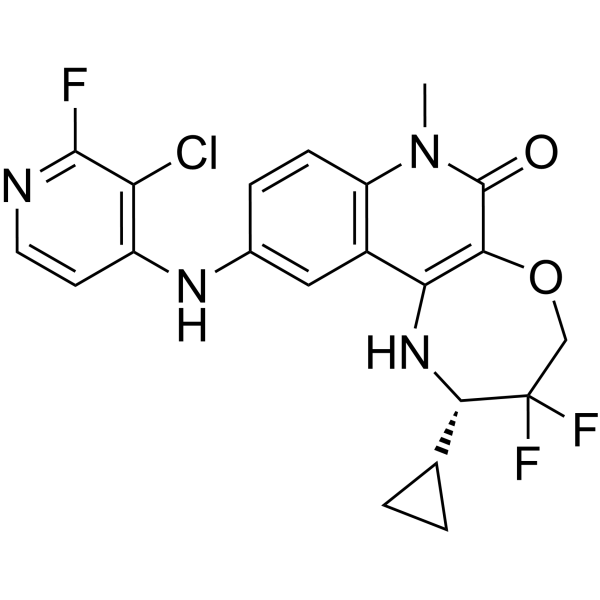
ClC1C(=NC=CC=1NC1C=CC2=C(C=1)C1=C(C(N2C)=O)OCC([C@H](C2CC2)N1)(F)F)F
|
| InChi Key |
MCRHRRIGPAVFNY-SFHVURJKSA-N
|
| InChi Code |
InChI=1S/C21H18ClF3N4O2/c1-29-14-5-4-11(27-13-6-7-26-19(23)15(13)22)8-12(14)16-17(20(29)30)31-9-21(24,25)18(28-16)10-2-3-10/h4-8,10,18,28H,2-3,9H2,1H3,(H,26,27)/t18-/m0/s1
|
| 化学名 |
(2S)-10-[(3-chloro-2-fluoropyridin-4-yl)amino]-2-cyclopropyl-3,3-difluoro-7-methyl-2,4-dihydro-1H-[1,4]oxazepino[2,3-c]quinolin-6-one
|
| 别名 |
CCT374705; 2640647-90-9; SCHEMBL23248186
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 本产品在运输和储存过程中需避光。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: 100 mg/mL (221.81 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 2.5 mg/mL (5.55 mM) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 悬浮液;超声助溶。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (5.55 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL 澄清 DMSO 储备液加入到 900 μL 玉米油中并混合均匀。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.2181 mL | 11.0904 mL | 22.1808 mL | |
| 5 mM | 0.4436 mL | 2.2181 mL | 4.4362 mL | |
| 10 mM | 0.2218 mL | 1.1090 mL | 2.2181 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。