| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| Other Sizes |
|
| 靶点 |
ULK1 (IC50 = 1.6 nM); ULK2 (IC50 = 30 nM)
|
|---|---|
| 体外研究 (In Vitro) |
ULK-101 (0-5 μM) 以浓度依赖性方式抑制 U2OS 细胞中 BafA1 诱导的 LC3B-II 积累[1]。
•与SBI-0206965相比,ULK-101具有更高的效价和选择性。[1] •ULK-101抑制自噬囊泡的成核和周转。[1] •ULK-101使kras驱动的肺癌细胞对营养限制敏感。[1] •ULK-101是研究ULK1与自噬功能的重要分子工具。[1] |
| 酶活实验 |
激酶活性检测和IC50计算[1]
ULK1和ULK2的IC50数据使用10点IC50Profiler分析,在最高浓度为10 μM(4个重复)和1 μM(4个重复)时进行半对数稀释,曲线上大多数浓度为8个数据点。为了进行选择性分析,使用500 nM SBI-0206965、40 nM ULK-101或15 nM ULK-100对KinaseProfiler进行了两份野生型人激酶检测。对于每个激酶反应,使用ATP的Km浓度。从阴性对照井中计算剩余活性百分比和抑制百分比。对于选择性分析,通过将每个激酶的抑制百分比除以ULK1的抑制百分比来计算相对抑制。GraphPad Prism 7采用变斜率(四参数)非线性回归模型拟合曲线,分别采用100%和0%的上限和下限约束进行IC50测定。 |
| 细胞实验 |
免疫印迹[1]
细胞在冷冻裂解缓冲液中裂解[10 mM KPO4, 1 mM EDTA, 10 mM MgCl2, 5 mM EGTA, 50 mM双甘油磷酸,0.5% NP40, 0.1% Brij35, 0.1%脱氧胆酸钠,1 mM NaVO4, 5 mM NaF, 2 mM DTT和完全蛋白酶抑制剂],并通过SDS-PAGE分解蛋白质。图1使用手工浇注的10%丙烯酰胺凝胶,所有其他印迹使用预铸的BOLT 4-12% Bis-Tris Plus凝胶。将蛋白转移到硝化纤维素膜上(LC3印迹用PVDF膜),用一抗在4°C下过夜,然后在室温下用二抗检测1小时。通过增强化学发光检测蛋白质(图1C),或在Odyssey Classic或Odyssey Clx成像仪上成像和定量(所有其他印迹)。 荧光显微镜[1] 通过逆转录病毒转染表达该质粒的细胞,选择低表达的单克隆,获得了U2OS-EGFP-DFCP1单克隆细胞系。4室35 mm no,每室2万个细胞。1.5玻璃底皿,24小时。补充培养基,并用5 μM ULK101(或DMSO对照)和100 nM AZD8055(或DMSO对照)处理细胞。使用尼康Ti Eclipse显微镜在FITC通道中每隔30分钟采集一次图像,并将其封闭在笼式培养箱中,并在37°C下保持,加湿5% CO2。为了量化,使用NIS Elements对图像进行反卷积、平滑、顶帽变换(“检测峰”函数)和阈值(根据强度),以获得每个细胞中dfcp1阳性对象的数量。在图4C中,稳定表达ptfLC3B的U2OS细胞在no. 2上接种约48小时。在24孔皿中放置1.5个玻璃片。细胞用5 μM ULK-101(或DMSO对照)处理3小时。1.5小时后,培养基中添加100 nM BafA1(或等量DMSO)。细胞用4%甲醛固定,细胞核用Hoechst-33342染色。细胞在FITC(绿色)和DAPI(蓝色)通道成像。为了定量,使用NIS Elements对图像进行反卷积、顶帽变换(“检测峰”函数)和阈值(按强度),以获得每个细胞gfp - lc3阳性物体的数量。 ATG12免疫荧光[1] 用100 nM AZD8055或体积相当的DMSO加或不加5 μM ULK-101(或DMSO对照)处理U2OS细胞2.5小时。细胞用4%甲醛固定,用0.2% tritonx100在1xDPBS中渗透,用3%牛血清白蛋白(BSA)和5%山羊血清在1xDPBS中阻断,用抗atg12抗体(在阻断缓冲液中稀释1:100)在4°C下染色过夜。然后用af488结合的二抗(1:1000)在室温下染色1小时,细胞核用Hoechst-33342反染,盖玻片倒置到凝胶载玻片上。在尼康Ti Eclipse显微镜上,用60倍油物镜在FITC(绿色)和DAPI(蓝色)通道中对细胞进行成像。每种情况下对30-50个细胞进行成像,代表性图像如图3C所示。 克隆生存试验[1] 将细胞(U2OS或NSCLC)接种于组织培养处理过的96孔板上,每孔1000个细胞,接种于添加10%胎牛血清的rmi -1640培养基上。24小时后,抽吸培养基,用1倍DPBS冲洗孔,并用ULK-101浓度梯度(终浓度为100 μM、50 μM、25 μM、12.5 μM、6.25 μM、3.1 μM、1.6 μM、0.8 μM、0.4 μM或0 μM)的全培养基(FM)或Optistarve (OS)代替。两天后,抽吸培养基(和ULK-101),用1倍DPBS冲洗孔,所有孔都用FM代替。五天后,根据制造商的说明,使用发光细胞滴度- glo测定相对ATP水平 |
| 参考文献 | |
| 其他信息 |
In response to stress, cancer cells generate nutrients and energy through a cellular recycling process called autophagy, which can promote survival and tumor progression. Accordingly, autophagy inhibition has emerged as a potential cancer treatment strategy. Inhibitors targeting ULK1, an essential and early autophagy regulator, have provided proof of concept for targeting this kinase to inhibit autophagy; however, these are limited individually in their potency, selectivity, or cellular activity. In this study, we report two small molecule ULK1 inhibitors, ULK-100 and ULK-101, and establish superior potency and selectivity over a noteworthy published inhibitor. Moreover, we show that ULK-101 suppresses autophagy induction and autophagic flux in response to different stimuli. Finally, we use ULK-101 to demonstrate that ULK1 inhibition sensitizes KRAS mutant lung cancer cells to nutrient stress. ULK-101 represents a powerful molecular tool to study the role of autophagy in cancer cells and to evaluate the therapeutic potential of autophagy inhibition.[1]
Autophagy is a conserved recycling process that has emerged as a critical effector of both oncogenes and tumor suppressors and a potent regulator of cancer cell fate (Liu and Ryan, 2012, Rosenfeldt and Ryan, 2009). Although autophagy is carried out by the coordinated activity of more than 30 proteins, just a few are enzymes with clear drug-targeting potential. Among these is ULK1, which has garnered interest as a small molecule target given its essential and early role in the pathway. Here, we have presented ULK-101 as a potent and selective ULK1 inhibitor and demonstrated its ability to suppress autophagy in human cells.[1] ULK-101 joins at least six other ULK1 inhibitors reported since 2015. The Shokat laboratory has developed a series of ULK1-targeted compounds that have provided valuable insights into the structure of ULK1, despite limited selectivity and potency in cells (Lazarus et al., 2015, Lazarus and Shokat, 2015). Two other notable inhibitors were found by mining pharmaceutical data for compounds with activity against ULK1, analogous to our approach. SBI-0206965 was developed from a FAK inhibitor and shown to reduce Beclin 1 Ser15 phosphorylation in cells (Egan et al., 2015). This compound was reported as selective, based primarily on a large-scale competition binding assay; however, our direct comparison using in vitro kinase assays found ULK-101 to be considerably more selective than SBI-0206965. MRT68921, derived from a TBK1 inhibitor, inhibited ULK1 potently in vitro and strongly suppressed autophagy in cells, with 1 μM shown to block BafA1-induced LC3-II accumulation in nutrient-starved murine embryonic fibroblasts (Petherick et al., 2015). Although the authors screened 80 other kinases for inhibition by MRT68921, it is difficult to compare the selectivity profiles of MRT68921 and ULK-101. ULK-101 was screened against 327 kinases. Of interest, MRT68921 cross-reacts with AMPK, which may represent a therapeutic liability given the broad tumor suppressive functions of AMPK signaling. Interestingly, whereas AMPK was also inhibited by ULK-100 in vitro, it was spared by ULK-101 (Table S1). Finally, a study employing in silico screening and structure-activity relationship analyses identified potent indazole-derived ULK1 inhibitors, although their selectivity and activity in cells remains to be determined (Wood et al., 2017).[1] A major unresolved issue in the autophagy field concerns the genetic and environmental contexts in which autophagy promotes tumor growth and represents a therapeutic target. Here, we have used ULK-101 to show that nutrient-stressed cells may be particularly susceptible to ULK1 inhibition. SBI-0206965 was similarly found to increase cell death in nutrient-starved cells or in those with chemical mTORC1 inhibition (Egan et al., 2015). These findings are consistent with other studies in which autophagy inhibition was particularly effective in cells deprived of nutrients (Eng et al., 2016, Guo et al., 2016). Together, this suggests that nutrient depletion caused by rapid tumor growth may create a unique vulnerability to autophagy inhibition. Finally, although we found that several lung cancer cell lines with oncogenic KRAS were sensitive to ULK-101, future work is required to fully define the genetic backgrounds in which targeting ULK1 and autophagy will be effective.[1] Limitations of the Study Interest is mounting in developing novel therapeutics that can modulate the fundamental mechanisms of human disease, including autophagy. Despite encouraging research progress, only a limited number of compounds that target autophagy are developed beyond basic research. Accordingly, we aim to move these autophagy inhibitors through preclinical development. ULK-100 and ULK-101 have performed well in vitro, but these compounds require further validation in vivo to proceed with preclinical testing. In addition, ULK1 targeting as a therapeutic mechanism may not be effective in all genetic or environmental contexts, and further research is needed to identify when this strategy would be most effective. |
| 分子式 |
C22H16F4N4OS
|
|---|---|
| 分子量 |
460.45
|
| 精确质量 |
460.098
|
| 元素分析 |
C, 57.39; H, 3.50; F, 16.50; N, 12.17; O, 3.47; S, 6.96
|
| CAS号 |
2443816-45-1
|
| PubChem CID |
137628686
|
| 外观&性状 |
Light yellow to yellow solid powder
|
| 密度 |
1.5±0.1 g/cm3
|
| 折射率 |
1.684
|
| LogP |
3.38
|
| tPSA |
87.5Ų
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
8
|
| 可旋转键数目(RBC) |
5
|
| 重原子数目 |
32
|
| 分子复杂度/Complexity |
685
|
| 定义原子立体中心数目 |
1
|
| SMILES |
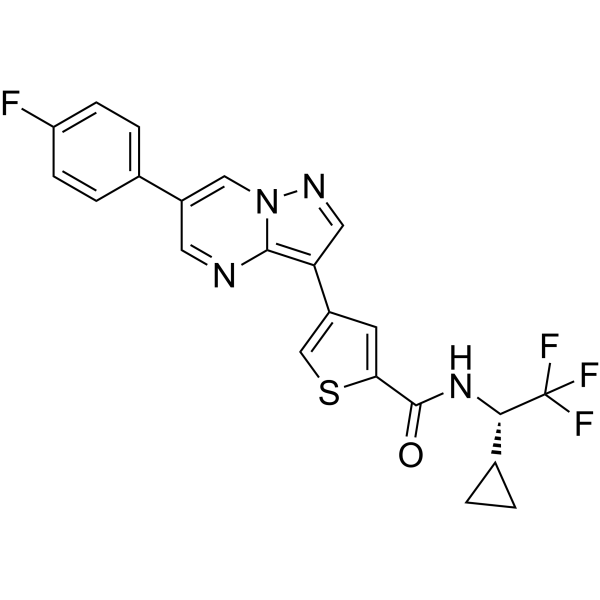
C1CC1[C@@H](C(F)(F)F)NC(=O)C2=CC(=CS2)C3=C4N=CC(=CN4N=C3)C5=CC=C(C=C5)F
|
| InChi Key |
PFZRXJIYAFANHP-IBGZPJMESA-N
|
| InChi Code |
InChI=1S/C22H16F4N4OS/c23-16-5-3-12(4-6-16)15-8-27-20-17(9-28-30(20)10-15)14-7-18(32-11-14)21(31)29-19(13-1-2-13)22(24,25)26/h3-11,13,19H,1-2H2,(H,29,31)/t19-/m0/s1
|
| 化学名 |
N-[(1S)-1-cyclopropyl-2,2,2-trifluoroethyl]-4-[6-(4-fluorophenyl)pyrazolo[1,5-a]pyrimidin-3-yl]thiophene-2-carboxamide
|
| 别名 |
ULK-101; 2443816-45-1; (S)-N-(1-cyclopropyl-2,2,2-trifluoroethyl)-4-(6-(4-fluorophenyl)pyrazolo[1,5-a]pyrimidin-3-yl)thiophene-2-carboxamide; N-[(1S)-1-Cyclopropyl-2,2,2-trifluoroethyl]-4-[6-(4-fluorophenyl)pyrazolo[1,5-a]pyrimidin-3-yl]thiophene-2-carboxamide; ULK101; CHEMBL4744680; SCHEMBL25395801; EX-A4693;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: 83.33 mg/mL (180.98 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (4.52 mM) (饱和度未知) in 10% DMSO + 40% PEG300 +5% Tween-80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80+,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.1718 mL | 10.8589 mL | 21.7179 mL | |
| 5 mM | 0.4344 mL | 2.1718 mL | 4.3436 mL | |
| 10 mM | 0.2172 mL | 1.0859 mL | 2.1718 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。