| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
Microbial Metabolite
|
|---|---|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
The present study investigated the fate of dihydroxyacetone (DHA) in an in vitro absorption study. In these studies, human ... skin penetration and absorption were determined over 24 or 72 hr in flow-through diffusion cells. ... For DHA, penetration studies found approximately 22% of the applied dose remaining in the skin (in both the stratum corneum and viable tissue) as a reservoir after 24 hr. Little of the DHA that penetrates into skin is actually available to become systemically absorbed. Metabolism / Metabolites Several bacteria use glycerol dehydrogenase to transform glycerol into dihydroxyacetone (DHA). DHA is subsequently converted into DHA phosphate (DHA-P) by an ATP- or phosphoenolpyruvate (PEP)-dependent DHA kinase. Listeria innocua possesses two potential PEP-dependent Dha kinases. One is encoded by 3 of the 11 genes forming the glycerol (gol) operon. This operon also contains golD (lin0362), which codes for a new type of DHA-forming NAD(+)-dependent glycerol dehydrogenase. The subsequent metabolism of DHA requires its phosphorylation via the PEP:sugar phosphotransferase system components enzyme I, HPr, and EIIA(DHA)-2 (Lin0369). P-EIIA(DHA)-2 transfers its phosphoryl group to DhaL-2, which phosphorylates DHA bound to DhaK-2. The resulting Dha-P is probably metabolized mainly via the pentose phosphate pathway, because two genes of the gol operon encode proteins resembling transketolases and transaldolases. In addition, purified Lin0363 and Lin0364 exhibit ribose-5-P isomerase (RipB) and triosephosphate isomerase activities, respectively. The latter enzyme converts part of the DHA-P into glyceraldehyde-3-P, which, together with DHA-P, is metabolized via gluconeogenesis to form fructose-6-P. Together with another glyceraldehyde-3-P molecule, the transketolase transforms fructose-6-P into intermediates of the pentose phosphate pathway. The gol operon is preceded by golR, transcribed in the opposite orientation and encoding a DeoR-type repressor. Its inactivation causes the constitutive but glucose-repressible expression of the entire gol operon, including the last gene, encoding a pediocin immunity-like (PedB-like) protein. Its elevated level of synthesis in the golR mutant causes slightly increased immunity against pediocin PA-1 compared to the wild-type strain or a pedB-like deletion mutant. |
| 毒性/毒理 (Toxicokinetics/TK) |
Interactions
... Consumption of dihydroxyacetone and pyruvate (DHP) increases muscle extraction of glucose in normal men. To test the hypothesis that these three-carbon compounds would improve glycemic control in diabetes the effect of DHP on plasma glucose concentration, turnover, recycling, and tolerance in 7 women with noninsulin-dependent diabetes /was evaluated/. The subjects consumed a 1,500-calorie diet (55% carbohydrate, 30% fat, 15% protein), randomly containing 13% of the calories as DHP (1/1) or Polycose (placebo; PL), as a drink three times daily for 7 days. On the 8th day, primed continuous infusions of [6-(3)H]-glucose and U-(14)C-glucose were begun at 05.00 hr, and at 09.00 hr a 3-hr glucose tolerance test (75 g glucola) was performed. Two weeks later the subjects repeated the study with the other diet. The fasting plasma glucose level decreased by 14% with DHP (DHP = 8.0 + or - 0.9 mmol/L; PL = 9.3 + or - 1.0 mmol/L, p less than 0.05) which accounted for lower postoral glucose glycemia (DHP = 13.1 + or - 0.8 mmol/L, PL = 14.7 + or - 0.8 mmol/L, p< 0.05). 6-(3)H-glucose turnover (DHP = 1.50 + or - 0.19 mg/kg-L/min, PL = 1.77 + or - 0.21 mmg/kg-L/min, p less than 0.05) and glucose recycling, the difference in 6-(3)H-glucose and U-(14)C-glucose turnover rates, decreased with DHP (DHP = 0.25 + or - 0.07 mg/kg-L/min, PL = 0.54 + or - 0.10 mg/kg-L/min, p< 0.05). Fasting and postoral glucose, plasma insulin, glucagon, and C peptide levels were unaffected by DHP. /Mixture of dihydroxyacetone and pyruvate/. Dihydroxyacetone (DHA) effectively antagonized the lethal effect of cyanide in mice and rabbits, particularly if administered in combination with thiosulfate. Oral DHA (2 and 4 g/kg) given to mice 10 min before injection (ip) of cyanide increased the LD50 values of cyanide from 5.7 mg/kg to 12 and 17.6 mg/kg, respectively. DHA prevented cyanide-induced lethality most effectively, if given orally 10-15 min before injection of cyanide. A combination of pretreatment with oral DHA (4 g/kg) and post-treatment with sodium thiosulfate (1 g/kg) increased the LD50 of cyanide by a factor of 9.9. Furthermore, DHA given intravenously to rabbits 5 min after subcutaneous injection of cyanide increased the LD50 of cyanide from 6 mg/kg to more than 11 mg/kg, while thiosulfate (1 g/kg) given intravenously 5 min after cyanide injection increased the LD50 of cyanide only to 8.5 mg/kg. DHA also prevented the convulsions that occurred after cyanide intoxication. Potassium cyanide (CN) intoxication in mice was found to be effectively antagonized by dihydroxyacetone (DHA), particularly if administered in combination with another CN antidote, sodium thiosulfate. Cyanide-induced convulsions were also prevented by DHA treatment, either alone or in combination with thiosulfate. Injection (ip) of DHA (2 g/kg) 2 min after or 10 min before CN (sc) increased LD50 values of CN (8.7 mg/kg) by factors of 2.1 and 3.0, respectively. Treatment with a combination of DHA and thiosulfate after CN increased the LD50 by a factor of 2.4. Pretreatment with a combination of DHA and thiosulfate (1 g/kg) increased the LD50 of CN to 83 mg/kg. Administration of alpha-ketoglutarate (2.0 g/kg), but not pyruvate, 2 min after CN increased the LD50 of CN by a factor of 1.6. Brain, heart and liver cytochrome oxidase activities were also measured following in vivo CN treatment with and without DHA. Pretreatment with DHA prevented the inhibition of cytochrome oxidase activity by CN and treatment with DHA after CN accelerated the recovery of cytochrome oxidase activity, especially in brain and heart homogenates ... |
| 参考文献 |
|
| 其他信息 |
Dihydroxyacetone is a ketotriose consisting of acetone bearing hydroxy substituents at positions 1 and 3. The simplest member of the class of ketoses and the parent of the class of glycerones. It has a role as a metabolite, an antifungal agent, a human metabolite, a Saccharomyces cerevisiae metabolite, an Escherichia coli metabolite and a mouse metabolite. It is a ketotriose and a primary alpha-hydroxy ketone.
Dihydroxyacetone is a metabolite found in or produced by Escherichia coli (strain K12, MG1655). Dihydroxyacetone has been reported in Arabidopsis thaliana, Homo sapiens, and other organisms with data available. Dihydroxyacetone is a metabolite found in or produced by Saccharomyces cerevisiae. A ketotriose compound. Its addition to blood preservation solutions results in better maintenance of 2,3-diphosphoglycerate levels during storage. It is readily phosphorylated to dihydroxyacetone phosphate by triokinase in erythrocytes. In combination with naphthoquinones it acts as a sunscreening agent. Mechanism of Action ...The toxicity of dihydroxyacetone appears to be due to its intracellular conversion to an aldehyde compound, presumably methylglyoxal, since the glyoxalase mutant becomes sensitive to dihydroxyacetone. Based on information that gldA is preceded in an operon by the ptsA homolog and talC gene encoding fructose 6-phosphate aldolase, this study proposes that the primary role of gldA is to remove toxic dihydroxyacetone by converting it into glycerol. Therapeutic Uses /The objective of this study was/ to evaluate the properties of dihydroxyacetone (DHA) in a new formulation for the treatment of vitiligo on exposed areas. ... Ten patients suffering from vitiligo affecting the face and/or hands /were treated/ with a newly introduced, commercially available self-bronzing cream containing DHA 5%. DHA was applied every second day. The characteristic pigmentation showed very satisfactory cosmetic results in 8 out of 10 patients after 2 weeks of treatment. The new DHA formulation is a practical and well-accepted treatment modality. /EXPL THER/ Dihydroxyacetone (DHA), a three-carbon sugar, is the browning ingredient in commercial sunless tanning formulations. ... In this work, the in vitro antifungal activity of dihydroxyacetone was tested against causative agents of dermatomycosis, more specifically against dermatophytes and Candida spp. The antifungal activity was determined by the broth microdilution method according to the Clinical and Laboratory Standards Institute guidelines for yeasts and filamentous fungi. The data obtained show that the fungicidal activity varied from 1.6 to 50 mg/mL. DHA seems to be a promising substance for the treatment of dermatomycosis because it has antifungal properties at the same concentration used in artificial suntan lotions. Therefore, it is a potential low-toxicity antifungal agent that may be used topically because of its penetration into the corneal layers of the skin. During seven months of a clinical trial in spring, summer, and fall, 30 UVA/B/Soret band-photosensitive patients used sequential topical applications of dihydroxyacetone (DHA) followed by naphthoquinone only at bedtime and received excellent photoprotection without a single therapeutic failure or loss of any patient to follow-up. Eighteen of the 30 patients extended the limits of their photoprotection repeatedly over a seven-month period to tolerate without sunburns six to eight hrs of midday sunlight under all kinds of occupational and recreational environmental conditions ... /EXPTL THER/ ... the protection with topical application of dihydroxyacetone (DHA) against solar UV-induced skin carcinogenesis in lightly pigmented hairless hr/hr C3H/Tif mice /was investigated/. ... Three groups of mice were UV-exposed four times a wk to a dose-equivalent of four times the standard erythema dose (SED), without or with application of 5 or 20% DHA only twice a week. Similarly, three groups of mice were treated with DHA and irradiated with a high UV dose (8 standard erythema dose), simulating a skin burn. Two groups (controls) were not irradiated, but either left untreated or treated with 20% DHA alone. The UV-induced skin pigmentation by melanogenesis could easily be distinguished from DHA-induced browning and was measured by a non-invasive, semi-quantitative method. Application of 20% DHA reduced by 63% the pigmentation produced by 4 standard erythema dose, however, only by 28% the pigmentation produced by 8 standard erythema dose. Furthermore, topical application of 20% DHA significantly delayed the time to appearance of the first tumor >or=1mm (P=0.0012) and the time to appearance of the third tumor (P=2 x 10(-6)) in mice irradiated with 4 standard erythema dose. However, 20% DHA did not delay tumor development in mice irradiated with 8 standard erythema dose. Application of 5% DHA did not influence pigmentation or photocarcinogenesis. /EXPTL THER/ ... Consumption of dihydroxyacetone and pyruvate (DHP) increases muscle extraction of glucose in normal men. To test the hypothesis that these three-carbon compounds would improve glycemic control in diabetes the effect of DHP on plasma glucose concentration, turnover, recycling, and tolerance in 7 women with noninsulin-dependent diabetes /was evaluated/. The subjects consumed a 1,500-calorie diet (55% carbohydrate, 30% fat, 15% protein), randomly containing 13% of the calories as DHP (1/1) or Polycose (placebo; PL), as a drink three times daily for 7 days. On the 8th day, primed continuous infusions of [6-(3)H]-glucose and [U-(14)C]-glucose were begun at 05.00 hr, and at 09.00 hr a 3-hr glucose tolerance test (75 g glucola) was performed. Two weeks later the subjects repeated the study with the other diet. The fasting plasma glucose level decreased by 14% with DHP (DHP = 8.0 + or - 0.9 mmol/L; PL = 9.3 + or - 1.0 mmol/L, p less than 0.05) which accounted for lower postoral glucose glycemia (DHP = 13.1 + or - 0.8 mmol/L, PL = 14.7 + or - 0.8 mmol/L, p less than 0.05). [6-(3)H]-glucose turnover (DHP = 1.50 + or - 0.19 mg/kg-L/min, PL = 1.77 + or - 0.21 mmg/kg-L/min, p less than 0.05) and glucose recycling, the difference in [6-(3)H]-glucose and [U-(14)C]-glucose turnover rates, decreased with DHP (DHP = 0.25 + or - 0.07 mg/kg-L/min, PL = 0.54 + or - 0.10 mg/kg-L/min, p less than 0.05). Fasting and postoral glucose, plasma insulin, glucagon, and C peptide levels were unaffected by DHP. /Mixture of dihydroxyacetone and pyruvate/. |
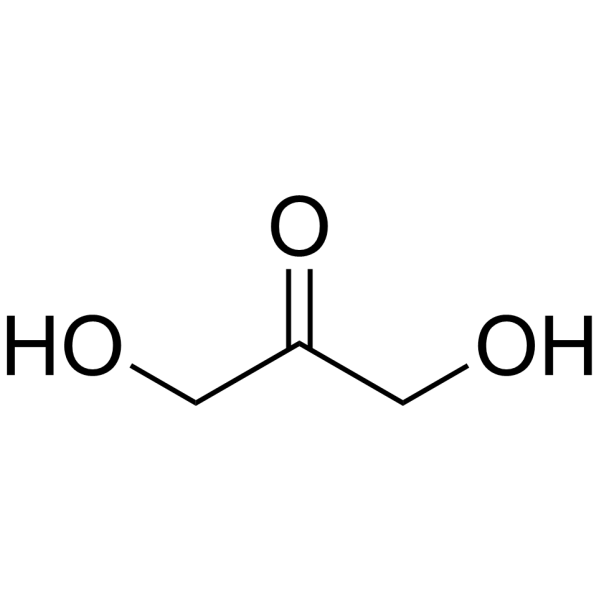
| 分子式 |
C3H6O3
|
|---|---|
| 分子量 |
90.08
|
| 精确质量 |
90.031
|
| CAS号 |
96-26-4
|
| 相关CAS号 |
26776-70-5
|
| PubChem CID |
670
|
| 外观&性状 |
White to off-white solid
|
| 密度 |
1.3±0.1 g/cm3
|
| 沸点 |
213.7±15.0 °C at 760 mmHg
|
| 熔点 |
75-80 °C
|
| 闪点 |
97.3±16.9 °C
|
| 蒸汽压 |
0.0±0.9 mmHg at 25°C
|
| 折射率 |
1.455
|
| LogP |
-0.78
|
| tPSA |
57.53
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
3
|
| 可旋转键数目(RBC) |
2
|
| 重原子数目 |
6
|
| 分子复杂度/Complexity |
44
|
| 定义原子立体中心数目 |
0
|
| SMILES |
O=C(CO)CO
|
| InChi Key |
RXKJFZQQPQGTFL-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C3H6O3/c4-1-3(6)2-5/h4-5H,1-2H2
|
| 化学名 |
1,3-dihydroxypropan-2-one
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO :~100 mg/mL (~1110.12 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (27.75 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (27.75 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (27.75 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 11.1012 mL | 55.5062 mL | 111.0124 mL | |
| 5 mM | 2.2202 mL | 11.1012 mL | 22.2025 mL | |
| 10 mM | 1.1101 mL | 5.5506 mL | 11.1012 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。