| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 100mg |
|
||
| 500mg |
|
||
| Other Sizes |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
... Seven male rats were used /in a metabolic study/; one received only non-labeled propoxur; the others received 1 mg/kg (14)C-labeled (ring) with sacrifice at 1, 4, 8, 24, 48, and 72 hr. The rats were sagittally sectioned and exposed (29-124 days) in direct contact with X-ray film. At 1 hour, radioactivity was detectable in all organs (particularly intestines) except the bone. After 24 hr, there were high concentrations of radioactivity in the gastrointestinal tract and bladder, as well as the mucous membranes of the pharyngeal region. At 48 and 72 hr, some radioactivity was still detectable in the liver, kidneys and mucous membranes of the pharyngeal system. Propoxur (and/or its metabolites) was shown to be distributed via the lymph system. Two studies ... provide information on the dermal adsorption of propoxur in humans and rats. In the human study, six individuals received a single intravenous dose of (14)C-propoxur, 1 Ci/mL. Total urine was collected for five days post-dose and the percent of radiolabeled-dose excreted in the urine was determined. Subsequently the same six individuals received a single dermal dose of (14)C-propoxur at 4 ug/sq cm for an exposure period of 24 hr. Total urine was collected for five days post-dose and the percent of radiolabeled-dose excreted in the urine was determined. The radiolabel excreted was corrected for the 81.8% of label excreted following the iv dose. Corrected total excretion was 19.6 percent of the dermally administered dose. In the rat study, four doses (0.648, 6.91, 69.5, and 692 ug/sq cm) were administered /to rats (strain and sex not given)/ for durations of 0.5, 1, 2, 4, 8, and 24 hr. Test material was administered in ethanol, a solvent which can increase the absorption of a dissolved chemical. Since percent absorption decreases in a nonlinear manner with dose, the absorption from the dose of 6.91 ug/sq cm (the nearest dose to that administered in the human study) was selected for comparison with the human study. The results indicate (for durations 0.5, 1, 2, 4, 8, and 32 hr) a total of 7.88, 10.2, 17.9, 23.2, and 32.5% absorption, respectively. The percent absorbed in the rat study exceeds the percent absorbed in the human study for exposure durations of 8 and 24 hr. This is expected, even without the addition of ethanol, as rat skin is more permeable than human skin. Alternatively, the use of acetone in the human study would show an expected increase of propoxur penetration. In a dermal absorption study with rats, a mixture of 50% ethanol and 50% water was used as a solvent, with doses of 0.648, 6.91, 69.5, or 692 ug/sq cm (corresponding nominal doses: 0.009875, 0.105, 1.0625, and 10.5 mg, respectively) radiolabeled propoxur. The highest values for absorption (50 to 64.9%) were observed with the two lowest dose levels, with the highest percentages of radioactivity (0.1-0.18%) in the blood occurring at these dose levels at 0.5 to 1.0 hr after dosage. Because propoxur was applied in a mixture of ethanol and water, the values obtained for dermal absorption were probably somewhat higher than if water alone had been the solvent. Like houseflies, rat ... degrades arpocarb... with 30% of applied dose expired as CO2 within 48-hr... For more Absorption, Distribution and Excretion (Complete) data for Propoxur (15 total), please visit the HSDB record page. Metabolism / Metabolites In a metabolism study, the following metabolites were identified in the urine of rats which had been fed 8000 ppm propoxur for 13 weeks: M1 = 1,2-dihydoxybenzene (= catechol); M2 = 2-isopropoxyphenol; M3 = 2-hydroxyphenyl methylcarbamate; M4 = 2- isopropoxyphenylcarbamic acid; M5 = isopropoxyphenyl-hydroxy(-) methylcarbamate; M6 = 2-isopropoxy-5-hydroxyphenyl-methylcarbamate; M7 = 2-isopropoxy-5-hydroxyphenyl carbamic acid; M8 = 2-isopropoxy-5-hydroxyphenylhydroxymethyl carbamate; M9 = 1,5-dihydroxy-2-isopropoxybenzene. In additional studies, M6 (= 2-isopropoxy-5-hydroxyphenylmethylcarbamate) was identified as a principle metabolite in hamsters, mice, and humans. The nitrosated compound M9A ( = 1-hydroxy-2- isopropoxy-4-nitrobenzene) has been identified as a metabolite in rats and mice, the rhesus monkey, and humans. Evidence from the human study suggests that M9A is synthesized in the stomach. Groups of 5 female rats received 50, 250, or 500 ppm unlabeled propoxur in their diets for 5 months, then received (by oral gavage) a single dose of 1 mg/kg radiolabelled material. Urine samples taken in the period from 0 to 24 hr after dosage had 87.9 to 99.8% of the total radiolabel; by thin layer chromatography it was found that 97-98% of the activity remained at the origin, and was contained in conjugated metabolites and/or extremely polar metabolites of unknown structure. By enzymatic cleavage 80-86% of the activity was identified as specific metabolites including M1, M2, M3, M4, M5, M6, M7, M8, as well as M6CII (= 2-isopropoxy-5-hydroxyphenyl carbamic acid), MS3 (= 2-isopropoxy-5-hydroxyphenyl-hydroxymethyl carbamate, and M7A (= 2- isopropoxy-3-hydroxyphenyl-methyl carbamate). In ... studies with ... Musca domestica l ... /metabolites were/ (in order of decr amt): 5-hydroxy-2-isopropoxyphenyl methylcarbamate, 2-hydroxyphenyl methylcarbamate & acetone, 2-isopropoxyphenyl n-hydroxymethylcarbamate, & 2-isopropoxyphenyl carbamate. There were 6 or more addnl unidentified cmpd. When the tert-carbon of 2-isopropoxy group of propoxur is oxidized to form a hemiketal, mono-N-methylcarbamoylcatechol is produced as a hydrolyzed metabolite that can normally be detected as 2-isopropoxyphenol by hydrolysis of the carbamoyl ester linkage, although this phenol has still not been detected. The 5-position of the phenyl ring is selectively metabolized in insects and their microsomes. For more Metabolism/Metabolites (Complete) data for Propoxur (7 total), please visit the HSDB record page. The carbamates are hydrolyzed enzymatically by the liver; degradation products are excreted by the kidneys and the liver. (L793) Biological Half-Life Following a single oral dose in rats, peak circulating & tissue concn of the major metabolite, isopropoxyl phenol, were achieved at 30 to 60 minutes, & the parent propoxur is eliminated very quickly (half-life = 11-15 min). |
|---|---|
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
Propoxur is a cholinesterase or acetylcholinesterase (AChE) inhibitor. Carbamates form unstable complexes with chlolinesterases by carbamoylation of the active sites of the enzymes. This inhibition is reversible. A cholinesterase inhibitor suppresses the action of acetylcholine esterase. Because of its essential function, chemicals that interfere with the action of acetylcholine esterase are potent neurotoxins, causing excessive salivation and eye-watering in low doses. Headache, salivation, nausea, vomiting, abdominal pain and diarrhea are often prominent at higher levels of exposure. Acetylcholine esterase breaks down the neurotransmitter acetylcholine, which is released at nerve and muscle junctions, in order to allow the muscle or organ to relax. The result of acetylcholine esterase inhibition is that acetylcholine builds up and continues to act so that any nerve impulses are continually transmitted and muscle contractions do not stop. Toxicity Data LC50 (rat) = 1,440 mg/m3/1h Interactions ... The effects of subchronic per os exposures to cadmium chloride (CdCl(2)) and ... propoxur (Pr), were investigated in male Wistar rats on general toxicological (body weight gain, relative organ weights) hematological (RBC, WBC, Ht, MCV, cell content of the femoral bone marrow) immune function (plaque forming cell (PFC) assay, delayed type hypersensitivity (DTH) reaction) and neurotoxicological (spontaneous and stimulus-evoked cortical activity, nerve conduction velocity) parameters. The animals were treated for 4, 8 and 12 weeks with 6.43 mg/kg CdCl(2), 8.51 mg/kg Pr, or with a combination of 6.43 mg/kg CdCl(2)+0.851 mg/kg Pr or 8.51 mg/kg Pr+1.61 mg/kg CdCl(2). Cadmium exposure affected the relative thymus, liver, and adrenal weight, RBC count, hematocrit and MCV, and there was an increase in nerve conduction velocity and a decrease in the cortical evoked potential latency. Pr induced a decrease in thymus weight, had some effect on the liver weight but none on the electrophysiological parameters. A significant interaction between Cd and Pr was detected by the following parameters: RBC, Ht, PFC, and nerve conduction velocity ... The toxicity of ... propoxur decreased in rats given Aroclor 1242. ... The effect of Withania somnifera (Ashwagandha), a widely used herbal drug possessing anti-stress and immunomodulatory properties, was studied on propoxur-induced acetylcholine esterase inhibition and impairment of cognitive function in rats. Male Wistar rats were divided into four groups. Group I was treated with olive oil and served as control. Group II was administered orally with propoxur (10 mg/kg bw) in olive oil, group III received a combination of propoxur (10 mg/kg bw) and W. somnifera (100 mg/kg bw) suspension, and group IV W. somnifera (100 mg/kg bw) only. All animals were treated for 30 days. Cognitive behavior was assessed by transfer latency using elevated plus maze. Blood and brain acetylcholine esterase (AChE) activity was also assessed. Oral administration of propoxur (10 mg/kg bw) resulted in a significant reduction of brain and blood AChE activity. A significant prolongation of the acquisition as well as retention transfer latency was observed in propoxur-treated rats. Oral treatment of W. somnifera exerts protective effect and attenuates AChE inhibition and cognitive impairment caused by sub-chronic exposure to propoxur. The effect of melatonin, a major secretory product of the pineal gland, in attenuation of propoxur -induced modulation of cell-mediated immune (CMI) response was studied in rats. Male Wistar albino rats were exposed to propoxur ... orally (10 mg/kg) and/or melatonin (10 mg/kg) orally for 4 weeks. CMI was measured by delayed-type hypersensitivity (DTH), leukocyte and macrophage migration inhibition (LMI and MMI) responses and estimation of cytokines TNF-alpha and IFN-gamma levels. Rats exposed to propoxur for 4 weeks showed significant decrease in DTH, LMI and MMI responses. Propoxur also suppressed TNF-alpha and IFN-gamma production significantly. Administration of melatonin alone caused a significant increase in DTH response. Although there were no changes in the LMI and MMI response, the cytokine levels were significantly increased, as compared to control. Co-administration of melatonin along with propoxur significantly nullified the effect of the pesticide on the CMI response, except DTH and reversed levels of cytokines to near control/normal values ... For more Interactions (Complete) data for Propoxur (6 total), please visit the HSDB record page. Non-Human Toxicity Values LD50 Rat acute oral 95 to 104 mg/kg LD50 Rat (male) oral 83 mg/kg LD50 Rat (female) oral 86 mg/kg LD50 Rat ip 30 mg/kg For more Non-Human Toxicity Values (Complete) data for Propoxur (15 total), please visit the HSDB record page. |
| 其他信息 |
Propoxur can cause cancer according to The Environmental Protection Agency (EPA).
Propoxur is a white to tan crystalline powder with a faint, characteristic odor. Used as an insecticide. (NIOSH, 2024) Propoxur is a carbamate ester that is phenyl methylcarbamate substituted at position 2 by a propan-2-yloxy group. It has a role as an EC 3.1.1.7 (acetylcholinesterase) inhibitor, a carbamate insecticide, an acaricide and an agrochemical. It is a carbamate ester and an aromatic ether. It is functionally related to a methylcarbamic acid and a 2-isopropoxyphenol. Propoxur is an insecticide used to control cockroaches, flies, mosquitoes, and lawn and turf insects. Acute (short-term) exposure of humans to propoxur by ingestion leads to cholinesterase inhibition of red blood cells, with mild cholinergic symptoms including blurred vision, nausea, vomiting, sweating, and tachycardia; however, the effects are transient. Chronic (long-term) inhalation exposure has resulted in depressed cholinesterase levels, headaches, vomiting, and nausea in humans. Chronic ingestion studies in animals have reported depressed cholinesterase levels, depressed body weight, effects to the liver and bladder, and a slight increase in neuropathy. No information is available on the reproductive, developmental, or carcinogenic effects of propoxur in humans. Mixed results are available from cancer studies of propoxur in animals. EPA has not classified propoxur for carcinogenicity. Propoxur is a synthetic carbamate, aromatic ether compound, and acetylcholinesterase inhibitor that is used as a pesticide. It is characterized as a toxic, white to tan crystalline solid with a faint odor, and exposure occurs by inhalation, ingestion, or contact. Propoxur is a carbamate pesticide. Carbamate pesticides are derived from carbamic acid and kill insects in a similar fashion as organophosphate insecticides. They are widely used in homes, gardens and agriculture. The first carbamate, carbaryl, was introduced in 1956 and more of it has been used throughout the world than all other carbamates combined. Because of carbaryl's relatively low mammalian oral and dermal toxicity and broad control spectrum, it has had wide use in lawn and garden settings. Most of the carbamates are extremely toxic to Hymenoptera, and precautions must be taken to avoid exposure to foraging bees or parasitic wasps. Some of the carbamates are translocated within plants, making them an effective systemic treatment. (L795) A carbamate insecticide. Mechanism of Action /A/ review of studies /was undertaken/ to evaluate mechanisms of rat urinary bladder tumors ... Propoxur or a metabolite was postulated to operate on or with growth factors to elicit hyperplasia and eventual tumor development. Since some propoxur metabolites are phenols, and since some phenols share with propoxur the property of being urinary bladder oncogens only at extremely high dose levels, threshold effects may be present. Low urinary pH was noted to inhibit binding of epidermal growth factor, which is abundant in rat urine. This is consistent with rat studies, in which pH reduction markedly reduced the extent of hyperplasia. Perspectives offered in this discussion suggest that propoxur-induced tumors in rats may not be relevant to man, but the "possible adverse effect" designation remains until more definitive evidence can be obtained. Carbamylation of acetylcholinesterase produces accumulation of acetylcholine and the picture of muscarinic and nicotinic poisoning. Spontaneous hydrolysis of the carbamate-cholinesterase complex occurs in vivo, leading to the disappearance of clinical effects within 24 hours. Penetration of the blood-brain barrier by the carbamates is insignificant; for this reason, few CNS symptoms occur. Propoxur inhibits cholinesterase & this effect is apparently the basis of its toxic action. Therapeutic Uses Medication (Vet): Effective against fleas and ticks on cattle, horse, cats, and dogs ... and sarcoptic mange of cattle ... Protection against ticks appears to wane after 1 week. |
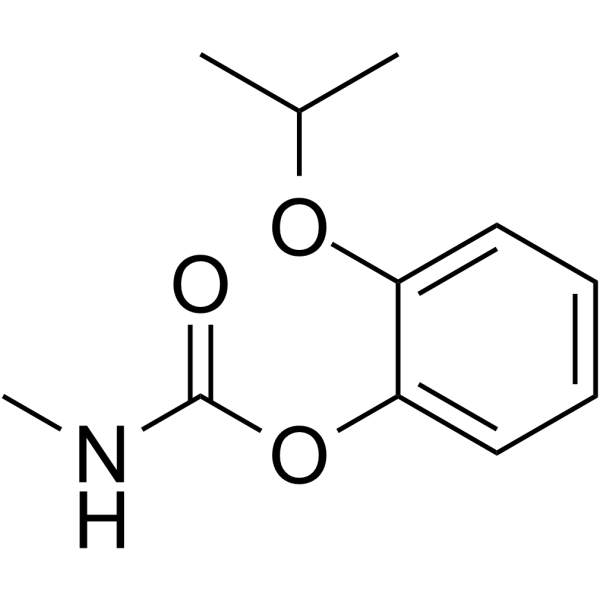
| 分子式 |
C11H15NO3
|
|---|---|
| 分子量 |
209.25
|
| 精确质量 |
209.105
|
| CAS号 |
114-26-1
|
| 相关CAS号 |
Propoxur-d3;1219798-56-7
|
| PubChem CID |
4944
|
| 外观&性状 |
Minute crystals
White, crystalline powder White to tan, crystalline solid |
| 密度 |
1.1±0.1 g/cm3
|
| 沸点 |
327.2±44.0 °C at 760 mmHg
|
| 熔点 |
91°C
|
| 闪点 |
151.7±28.4 °C
|
| 蒸汽压 |
0.0±0.7 mmHg at 25°C
|
| 折射率 |
1.499
|
| LogP |
2.56
|
| tPSA |
47.56
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
3
|
| 可旋转键数目(RBC) |
4
|
| 重原子数目 |
15
|
| 分子复杂度/Complexity |
206
|
| 定义原子立体中心数目 |
0
|
| SMILES |
CC(C)OC1=CC=CC=C1OC(NC)=O
|
| InChi Key |
ISRUGXGCCGIOQO-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C11H15NO3/c1-8(2)14-9-6-4-5-7-10(9)15-11(13)12-3/h4-8H,1-3H3,(H,12,13)
|
| 化学名 |
(2-propan-2-yloxyphenyl) N-methylcarbamate
|
| 别名 |
AI3-25671; Baygon; Propoxur
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ≥ 100 mg/mL (~477.92 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (9.94 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (9.94 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 悬浮液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (9.94 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 4.7790 mL | 23.8949 mL | 47.7897 mL | |
| 5 mM | 0.9558 mL | 4.7790 mL | 9.5579 mL | |
| 10 mM | 0.4779 mL | 2.3895 mL | 4.7790 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。