| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 体外研究 (In Vitro) |
体外活性:补骨脂素抑制 MCF-7/ADR 细胞的增殖,如 G0/G1 期阻滞所示,而不是促进细胞凋亡。补骨脂素通过抑制 ATP 酶活性而不是减少 P-gp 表达来逆转 MDR(多药耐药性)。补骨脂素可能通过抑制 NF-κB 的激活来抑制 EMT,从而抑制 MCF-7/ADR 细胞的迁移能力。补骨脂素是一种光活性化合物,当被长波紫外线激活时,很容易烷基化 DNA。用低浓度补骨脂素(<10.75 且=抑制=高=>21.5 µM)处理的MCF-7/ADR 细胞的增殖得到显着促进。补骨脂素可以抑制乳腺癌的转移。补骨脂素介导多种细胞过程,包括细胞死亡、增殖、炎症和迁移。细胞测定:通过MTT测定来测量补骨脂素对细胞增殖的影响。 MCF-10A和MCF-7/ADR细胞在96孔板中以每孔2×104个细胞的细胞密度培养48小时。然后除去培养基并更换为含有不同浓度补骨脂素(0、21.5、43.0、64.5、86.0、107.5 μM)的新鲜培养基48小时。阴性对照组的细胞与补充有0.1%二甲基亚砜(DMSO)的RPMI-1640培养基一起孵育。将细胞与 10 µL MTT (5 mg/mL) 一起孵育 4 小时,然后弃去培养基并添加 200 µL DMSO。晶体完全溶解后,用酶标仪在490 nm处测定分光光度吸光度。
|
|---|---|
| 体内研究 (In Vivo) |
补骨脂素已被认为是多种肿瘤中的肿瘤抑制因子。补骨脂素可改善雌性和雄性小鼠性激素缺乏引起的骨质疏松症。它通过刺激骨髓间充质干细胞的成骨细胞分化,对卵巢切除诱导的骨质疏松大鼠具有抗骨质疏松作用。
|
| 细胞实验 |
MTT法用于测量补骨脂素对细胞增殖的影响。 MCF-10A 和 MCF-7/ADR 细胞在 96 孔板中以每孔 2×104 的细胞密度培养 48 小时。之后,取出培养基并更换为具有不同补骨脂素含量(0、21.5、43.0、64.5、86.0 和 107.5 μM)的新培养基,培养 48 小时。阴性对照组的细胞在添加有0.1%二甲基亚砜(DMSO)的RPMI-1640培养基中培养。将细胞与 10 µL MTT (5 mg/mL) 一起孵育 4 小时后,弃去培养基并添加 200 µL DMSO。晶体完全溶解后,使用酶标记仪在 490 nm 处测量分光光度吸光度。
|
| 动物实验 |
ICR mice
10 mg/kg and 20 mg/kg intragastrically |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
OBJECTIVE: To investigate the nasal absorption regularities of psoralen and isopsoralen of different concentrations. METHOD: Building an experimental model of rat in situ nasal recirculation and determining the contents of psoralen and isopsoralen by HPLC. RESULT: The nasal absorption of psoralen and isopsoralen fitted in with zero order kinetics, getting saturated with the increase of concentration. CONCLUSION: A suitable concentration is necessary for the preparation of nasal remedies psoralen and isopsoralen. Metabolism / Metabolites Psoralen has known human metabolites that include 5,7,11-Trioxatetracyclo[8.4.0.03,8.04,6]tetradeca-1,3(8),9,13-tetraen-12-one. |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION AND USE: Psoralen is a solid. Psoralen photochemotherapy (PUVA) is the combined treatment of skin disorders with a photosensitizing drug (Psoralen) and UltraViolet A radiation. The introduction of PUVA therapy has arguably been the most important development in dermatology over the past 30 years. HUMAN STUDIES: Exposure to more than 350 PUVA treatments greatly increases the risk of squamous cell carcinoma. Exposure to fewer than 150 PUVA treatments has, at most, modest effects on squamous cell carcinoma risk. Even high-dose exposure to PUVA does not greatly increase basal cell carcinomaa risk. The risks of squamous cell carcinom in long-term PUVA-treated patients should be considered in determining the risk of this therapy relative to other treatments for severe psoriasis. Psoralen ultraviolet B radiation (PUVB) can contribute to blue vitiligo. Psoralen can generate a very unique type of DNA damage, namely ICL (interstrand cross-link). An ICL can severely block DNA replication and transcription and cause programmed cell death. It is proposed that PUVA therapy conditions are more favorable for the formation of immunosuppressive rather than membrane-damaging psoralen photooxidation products. Psoralen inhibited the viability of normal human liver L02 cells in vitro by inducing S-phase arrest. In addition, psoralen upregulated cyclin E1 and p27 protein levels in these cells. ANIMAL STUDIES: Mice were administered psoralen intragastrically at doses of 400 mg/kg or 800 mg/kg, and were sacrificed 24 hr after treatment. Changes in various hepatotoxicity indicators demonstrated that psoralen can cause mild liver injury in mice. In addition, psoralen upregulated cyclin E1 and p27 protein levels in mouse livers. In rats, the liver was the target of toxicity of psoralen. Multivariate analysis identified 7 metabolites in serum samples and 15 in liver samples as potential biomarkers in liver injury induced by psoralen. In addition, psoralen can cause a disturbance in amino acid metabolism, especially valine, leucine, and isoleucine biosynthesis in both serum and liver samples. Mutagenic activity of psoralen was studied in the HGPRT system on V79 Chinese hamster cells in culture. When activated by near-ultraviolet (NUV) light, were effective in inducing HGPRT mutants. Albino guinea pigs were treated with furocoumarin derivatives plus 320-400 NM UV radiation, and DNA was extracted from their epidermis. The electron microscopic assay was sensitive enough to put an upper limit of 1 crosslink/10X6 nucleotide pairs (80 cross-links/chromosome) for the low dose studies. Psoralen was demonstrated to exhibit in vitro inhibitory actions on monoamine oxidase (MAO) activities in rat brain mitochondria, preferentially inhibiting MAO-A activity over MAO-B activity. ECOTOXICITY STUDIES: The rRNA genes from fungi, plants, and animals give distinctly bimodal distributions of psoralen crosslinking, which has led to the suggestion that these genes might be largely devoid of nucleosomes when transcriptionally active. Chromatin structure of the multicopy rRNA and histone genes was studied during sea urchin early embryogenesis. The rRNA genes, which are weakly expressed, give a unimodal distribution of weak psoralen crosslinking, in contrast to the situation in all other organisms studied. The early histone genes were more accessible to psoralen crosslinking when active than inactive. The mechanism of action many furocoumarins is based on their ability to form photoadducts with DNA and other cellular components such as RNA, proteins, and several proteins found in the membrane such as phospholipases A2 and C, Ca-dependent and cAMPdependent protein-kinase and epidermal growth factor. Furocoumarins intercalate between base pairs of DNA and after ultraviolet-A irradiation, giving cycloadducts. (L579). Hepatotoxicity In open label trials, serum ALT or AST elevations occurred in 2% to 12% of subjects treated with methoxsalen and UV light. The elevations were usually mild-to-moderate in severity, asymptomatic and self-limited in course. Clinically apparent acute liver injury has also been reported with oral methoxsalen therapy, but only in isolated case reports including one instance attributed to topical methoxsalen therapy. The time to onset has ranged from 1 to 5 months, the typical latency being 6 to 8 weeks. The onset is generally insidious, with appearance of nausea and abdominal pain followed by jaundice. Fever occurs in some cases, but rash and eosinophilia are not common. The typical pattern of injury is hepatocellular. Most published cases of psoralen hepatotoxicity have been mild-to-moderate in severity, but severe jaundice and death from hepatic failure has been described in patients with preexisting cirrhosis who developed further acute liver injury attributed to methoxsalen. Most cases resolve within 6 to 8 weeks. Psoralen is also present in many herbal products used to treat various conditions including psoriasis and vitiligo. Case reports of acute liver injury have been reported with the use of seeds, powder and teas prepared from Psoralea corylifolia under various Chinese names such as Boh Gol Zhee, Xin Cu Hei Su and Qu Bai Ba Bu Gi Pian. Chemical analyses have shown the presence of psoralen in these products. The clinical features of these cases have resembled those attributed to methoxsalen with a latency of 1 to 2 months, a hepatocellular pattern of injury, absence of immunoallergic or autoimmune features, and self-limited course with recovery within 6 to 8 weeks. Likelihood score: C (probable rare cause of clinically apparent liver injury) Interactions BACKGROUND: The use of psoralen-UVA (PUVA) in patients of skin phototype I to II is limited by side effects of acute phototoxicity and possible long-term carcinogenesis. OBJECTIVE: We sought to assess oral Polypodium leucotomos (PL) extract in decreasing PUVA-induced phototoxicity of human skin on a clinical and histologic level. METHODS: A total of 10 healthy patients with skin phototypes II to III were exposed to PUVA alone (using 0.6 mg/kg oral 8-methoxypsoralen) and to PUVA with 7.5 mg/kg of oral PL. RESULTS: Clinically, phototoxicity was always lower in PL-treated skin after 48 to 72 hours (P<0.005), and pigmentation was also reduced 4 months later. Histologically, PL-treated skin showed a significant numeric reduction of sunburn cells (P=0.05), preservation of Langerhans cells (P< or =0.01), decrease of tryptase-positive mast cell infiltration (P<0.05), and decrease of vasodilation (P< or =0.01). No differences were found in Ki-67+ proliferating cells. CONCLUSIONS: PL is an effective chemophotoprotector against PUVA-induced skin phototoxicity and leads to substantial benefits of skin protection against damaging effects of PUVA as evidenced by histology. Chemotherapy is the recommended treatment for advanced-stage cancers. However, the emergence of multidrug resistance (MDR), the ability of cancer cells to become simultaneously resistant to different drugs, limits the efficacy of chemotherapy. Previous studies have shown that herbal medicine or natural food may be feasible for various cancers as potent chemopreventive drug. This study aims to explore the capablility of reversing the multidrug resistance of docetaxel (DOC)-resistant A549 cells (A549/D16) of psoralen and the underlying mechanisms. In this study, results showed that the cell viability of A549/D16 subline is decreased when treated with psoralen plus DOC, while psoralen has no effect on the cell proliferation on A549 and A549/D16 cells. Furthermore, mRNA and proteins levels of ABCB1 were decreased in the presence of psoralen, while decreased ABCB1 activity was also revealed by flow cytometry. Based on these results, we believe that psoralen may be feasible for reversing the multidrug resistance by inhibiting ABCB1 gene and protein expression. Such inhibition will lead to a decrease in ABCB1 activity and anti-cancer drug efflux, which eventually result in drug resistance reversal and therefore, sensitizing drug-resistant cells to death in combination with chemotherapeutic drugs. Furanocoumarin compound psoralen (PRN) is a major active ingredient found in herbaceous plants. PRN has been used for the treatment of various dermal diseases in China. We evaluated the inhibitory effect of PRN on cytochrome P450 2B6 (CYP2B6) and found that PRN induced a time-, concentration-, and NADPH-dependent inactivation of CYP2B6 with the values of KI and kinact being 110.2 uM and 0.200 min(-1), respectively. Ticlopidine, a CYP2B6 substrate, prevented the enzyme from the inactivation induced by PRN. Exogenous nucleophile glutathione (GSH) and catalase/superoxide dismutase showed limited protection of CYP2B6 from the inactivation. The estimated partition ratio of the inactivation was approximately 400. GSH trapping experiments indicates that an epoxide or/and gamma-ketoenal intermediate was formed in microsomal incubations with PRN. In summary, PRN was characterized as a mechanism-based inactivator of CYP2B6. Naturally occurring furanocoumarin compounds psoralen (PRN) and isopsoralen (IPRN) are bioactive constituents found in herbaceous plants. They are widely used as active ingredients in several Chinese herbal medicines. In this study, the CYP1A2 inhibitory potential of PRN and IPRN was investigated in rats in vitro and in vivo as well as in human liver microsomes. Both compounds exhibited reversible and time-dependent inhibition toward rat microsomal cyp1a2. The IC(50), k(inact), and K(I) values were 10.4 +/- 1.4 uM, 0.060 +/- 0.002 min(-1), and 1.13 +/- 0.12 uM for PRN, and 7.1 +/- 0.6 uM, 0.10 +/- 0.01 min(-1), and 1.95 +/- 0.31 uM for IPRN, respectively. In human liver microsomal incubations, potent reversible CYP1A2 inhibition was observed for both compounds, with IC(50) values of 0.26 +/- 0.01 uM and 0.22 +/- 0.03 uM for PRN and IPRN, respectively. However, time-dependent inhibition was only observed for IPRN, with kinact and KI values of 0.050 +/- 0.002 min(-1) and 0.40 +/- 0.06 uM, respectively. Coadministration with PRN or IPRN significantly inhibited cyp1a2 activity in rats, with the area under the curve (AUC) of phenacetin increasing more than 5-fold. Simcyp simulation predicted that PRN would cause 1.71- and 2.12-fold increases in the phenacetin AUC in healthy volunteers and smokers, respectively. IPRN, on the other hand, would result in 3.24- and 5.01-fold increases in phenacetin AUCs in healthy volunteers and smokers, respectively. These findings represent the first detailed report comparing the potential drug-drug interactions of PRN and IPRN, and provide useful information for balancing safe and efficacious doses of PRN and IPRN. For more Interactions (Complete) data for Psoralen (40 total), please visit the HSDB record page. |
| 参考文献 | |
| 其他信息 |
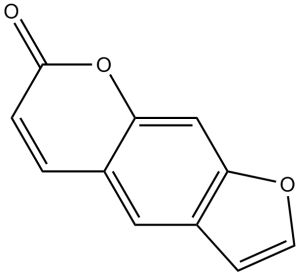
Psoralen is the simplest member of the class of psoralens that is 7H-furo[3,2-g]chromene having a keto group at position 7. It has been found in plants like Psoralea corylifolia and Ficus salicifolia. It has a role as a plant metabolite.
8-methoxsalen and 5-methoxsalen are furocoumarins referred to collectively as psoralens that have photosensitizing activity and are used orally and topically in conjunction with ultraviolet irradiation for the therapy of psoriasis and vitiligo. Psoralens have been linked to a low rate of transient serum enzyme elevations during therapy and to rare instances of clinically apparent acute liver injury. Psoralen has been reported in Ficus erecta var. beecheyana, Hoita macrostachya, and other organisms with data available. Psoralen is a furocoumarin that intercalates with DNA, inhibiting DNA synthesis and cell division. Psoralen is used in Photochemotherapy with high-intensity long-wavelength UVA irradiation. Psoralens are tricyclic furocumarins and have a strong tendency to intercalate with DNA base pairs. Irradiation of nucleic acids in the presence of psoralen with long wave UV (~360 nm) results in the 2+2 cyclo- addition of either of its two photoreactive sites with 5,6-carbon bonds of pyrimidines resulting in crosslinking double-stranded nucleic acids. Psoralen is found in carrot. Psoralen is found in common vegetables, e.g. parsnip, celery especially if diseased or `spoiled' Psoralen is a significant mutagen and is used for this purpose in molecular biology research. Psoralen has been shown to exhibit anti-proliferative, anti-allergenic and anti-histamine functions (A7781, A7782, A7782). Psoralen belongs to the family of Furanocoumarins. These are polycyclic aromatic compounds containing a furan ring fused to a coumarin moeity. A naturally occurring furocoumarin, found in PSORALEA. After photoactivation with UV radiation, it binds DNA via single and double-stranded cross-linking. See also: Angelica keiskei top (part of); Cullen corylifolium fruit (part of). Therapeutic Uses /EXPL THER/ Osteoporosis is a systemic skeletal disease, which is characterized by a systemic destruction of bone mass and microarchitecture. With life standard improved, the treatment of osteoporosis attracted more attention. The aim of this study is to verify the osteoprotective effect of psoralen and isopsoralen in females and males. Female and male mice were divided into 7 groups in this study: control group (sham-operation), model group (by ovariectomy or orchidectomy), positive control group (females given estradiol valerate; males given alendronate sodium), psoralen groups (10 mg/kg and 20 mg/kg), and isopsoralen groups (10 mg/kg and 20 mg/kg). After administration of psoralen and isopsoralen for 8 weeks, osteoporosis was ameliorated with increasing bone strength and improving trabecular bone microstructure as indicated by CT scan and pathology. Serum alkaline phosphatase (ALP), tartrate resistant acid phosphatase (TRACP), osteocalcin (OC), and C-terminal cross-linking telopeptides of type I collagen (CTX-1) were examined. Decreased TRACP and increased ALP/TRACP suggested restoring from bone destruction. These results suggest that psoralen and isopsoralen may be used as good natural compounds for the treatment of osteoporosis in males, as well as females. /EXPL THER/ This work investigates X-PACT (X-ray Psoralen Activated Cancer Therapy): a new approach for the treatment of solid cancer. X-PACT utilizes psoralen, a potent anti-cancer therapeutic with current application to proliferative disease and extracorporeal photopheresis (ECP) of cutaneous T Cell Lymphoma. An immunogenic role for light-activated psoralen has been reported, contributing to long-term clinical responses. Psoralen therapies have to-date been limited to superficial or extracorporeal scenarios due to the requirement for psoralen activation by UVA light, which has limited penetration in tissue. X-PACT solves this challenge by activating psoralen with UV light emitted from novel non-tethered phosphors (co-incubated with psoralen) that absorb x-rays and re-radiate (phosphoresce) at UV wavelengths. The efficacy of X-PACT was evaluated in both in-vitro and in-vivo settings. In-vitro studies utilized breast (4T1), glioma (CT2A) and sarcoma (KP-B) cell lines. Cells were exposed to X-PACT treatments where the concentrations of drug (psoralen and phosphor) and radiation parameters (energy, dose, and dose rate) were varied. Efficacy was evaluated primarily using flow cell cytometry in combination with complimentary assays, and the in-vivo mouse study. In an in-vitro study, we show that X-PACT induces significant tumor cell apoptosis and cytotoxicity, unlike psoralen or phosphor alone (p<0.0001). We also show that apoptosis increases as doses of phosphor, psoralen, or radiation increase. Finally, in an in-vivo pilot study of BALBc mice with syngeneic 4T1 tumors, we show that the rate of tumor growth is slower with X-PACT than with saline or AMT + X-ray (p<0.0001). Overall these studies demonstrate a potential therapeutic effect for X-PACT, and provide a foundation and rationale for future studies. In summary, X-PACT represents a novel treatment approach in which well-tolerated low doses of x-ray radiation are delivered to a specific tumor site to generate UVA light which in-turn unleashes both short- and potentially long-term antitumor activity of photo-active therapeutics like psoralen. /EXPL THER/ Mycosis fungoides with large-cell transformation is historically associated with a poor prognosis. Pediatric cases of mycosis fungoides with large-cell transformation are rare, with only three other cases reported in the literature. We present the first case of a child with almost complete remission of his mycosis fungoides with large-cell transformation shortly after administration of psoralen plus ultraviolet A, interferon-alfa, and localized radiation. /EXPL THER/ BACKGROUND: Isotretinoin has been used in combination with oral psoralen+UVA (PUVA) and narrowband UVB (NBUVB) for treating psoriasis, especially in women of child-bearing age. The efficacy of oral psoralen+sun exposure (PUVAsol) is comparable to that of PUVA. This study was planned to compare the efficacy of oral PUVAsol with that of the combination of oral isotretinoin and PUVAsol in patients with chronic plaque psoriasis. METHODS: Forty patients with psoriasis vulgaris were randomized to two groups. Group A (control group) received PUVAsol only. Group B (intervention group) received PUVAsol+isotretinoin (0.5 mg/kg/day). Psoriasis Area Severity Index (PASI) score was recorded at baseline and weeks 4, 8 and 12. Dermatology Life Quality Index was assessed at baseline and 12 weeks. The end point of the study was PASI 75 or 12 weeks, whichever came earlier. RESULTS: Thirty-five patients completed the study. There were statistically significant differences between the two study groups for the number of patients achieving the endpoint of PASI 75, PASI scores at the end of 12 weeks, mean duration to achieve PASI 75, number of PUVAsol sessions needed to achieve PASI75 and mean cumulative dosage of 8-methoxypsoralen needed to achieve PASI 75. CONCLUSION: The combination of isotretinoin with PUVAsol is more effective compared with PUVAsol alone for treating chronic plaque psoriasis. For more Therapeutic Uses (Complete) data for Psoralen (14 total), please visit the HSDB record page. Drug Warnings This study was aimed to alert the hazard of accidental adverse reactions of photochemotherapy (Psoralen-UVA or PUVA) that has been used in the treatment for some skin diseases and commercially for cosmetic tanning. Aside from the predictable side effects of PUVA such as erythema and itching, the accidental adverse reactions such as extensive burns could occasionally occur. Our observations indicated that six cases resulted from mistakes of medical personnel, and six other cases resulted from unsupervised mistakes of patients. The conditions that needed photochemotherapy were seven cases of vitiligo, three cases of psoriasis and two cases of tanning. The accidental overdose of UV radiation was about 3-10 times the empirically normal dose. Five of our patients were supposed to undergo topical PUVA, but they were irradiated at the dose of oral PUVA. One patient applied 8-methoxypsoralen (8-MOP) cream together with taking 5-methoxypsoralen (5-MOP) tablets for oral PUVA. Three other patients enjoyed sunbathing 1-3h shortly after finishing PUVA. A young couple chose 5-MOP to enhance tanning and sunbathed about 1h later. When another patient resumed PUVA in a 6-month cessation, he was exposed at a previous dose instead of a starting dose. Erythema and blisters of second degree burns developed in all our cases, 36-72h after PUVA, with 5-25% of body surface involved. Among the 12 patients, 3 were admitted and 9 were treated on an outpatient basis. All patients recovered in 1-3 weeks with no skin graft or no significant sequelae except post-inflammatory hyperpigmentation. POTENTIAL ADVERSE EFFECTS ON FETUS: Animal studies not performed. Unknown effect on humans. Should be given only if clearly needed. POTENTIAL SIDE EFFECTS ON BREAST-FED INFANT: None known whether excreted. Should be avoided. FDA Category: C (C = Studies in laboratory animals have revealed adverse effects on the fetus (teratogenic, embryocidal, etc.) but there are no controlled studies in pregnant women. The benefits from use of the drug in pregnant women may be acceptable despite its potential risks, or there are no laboratory animal studies or adequate studies in pregnant women.) /Psoralens/ /from table II/ Psoralen plus ultraviolet A irradiation (PUVA therapy) is commonly used for the management of vitiligo in which perifollicular repigmentation is the usual response pattern. However, excessive PUVA therapy may be associated with adverse effects. We report a case of generalized vitiligo that has been extensively treated with topical and systemic PUVA therapy for several years with the development of extensive and widespread stellate and irregularly shaped black and brown macules (lentigines). Interestingly, the lentigines were observed not only in the normally pigmented skin but also within the depigmented lesions that were lacking the perifollicular response pattern. The lesions developed in the exposed and unexposed skin areas. No evidence of skin malignancy was observed clinically and no melanocyte atypia was detected histopathologically. Cryotherapy may be used in the management of the lentigines; however, because of the extent of lesions this was impractical in our case. BACKGROUND: Changes in the appearance of the skin including actinic degeneration and pigmentary changes have been noted in patients treated with psoralen and UVA (PUVA). OBJECTIVE: Our purpose was to quantify risk factors for increased extent and progression of actinic degeneration and pigmentary changes in the skin of patients treated with PUVA. METHODS: On the basis of standardized dermatologic examination conducted in 1977 and 1998 of patients enrolled in the PUVA Follow Up Study, we assessed the prevalence of and changes in the extent of actinic degeneration and pigmentary abnormalities on the hands and buttocks. RESULTS: From 1977 to 1998, the prevalence of moderate or severe actinic degeneration increased from 15.6% to 60.5% on the hands and from 2.2% to 21.3% on the buttocks. During this same period, the prevalence of pigmentary changes of this degree increased from 15.6% to 58.6% on the hands and 12.6% to 24.7% on the buttocks. Extent of exposure to PUVA was the strongest predictor of an increased extent of clinical actinic degeneration or pigmentary change. CONCLUSION: Long-term exposure to PUVA is associated with persistent increases in actinic degeneration and pigmentary abnormalities of the skin on both usually sun-exposed and sun-protected sites. For more Drug Warnings (Complete) data for Psoralen (8 total), please visit the HSDB record page. |
| 分子式 |
C11H6O3
|
|
|---|---|---|
| 分子量 |
186.16
|
|
| 精确质量 |
186.031
|
|
| CAS号 |
66-97-7
|
|
| 相关CAS号 |
|
|
| PubChem CID |
6199
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 密度 |
1.4±0.1 g/cm3
|
|
| 沸点 |
362.6±27.0 °C at 760 mmHg
|
|
| 熔点 |
160-162 °C
|
|
| 闪点 |
173.1±23.7 °C
|
|
| 蒸汽压 |
0.0±0.8 mmHg at 25°C
|
|
| 折射率 |
1.667
|
|
| LogP |
1.67
|
|
| tPSA |
43.35
|
|
| 氢键供体(HBD)数目 |
0
|
|
| 氢键受体(HBA)数目 |
3
|
|
| 可旋转键数目(RBC) |
0
|
|
| 重原子数目 |
14
|
|
| 分子复杂度/Complexity |
284
|
|
| 定义原子立体中心数目 |
0
|
|
| InChi Key |
ZCCUUQDIBDJBTK-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C11H6O3/c12-11-2-1-7-5-8-3-4-13-9(8)6-10(7)14-11/h1-6H
|
|
| 化学名 |
furo[3,2-g]chromen-7-one
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (13.43 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: 2.5 mg/mL (13.43 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (13.43 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 5.3717 mL | 26.8586 mL | 53.7172 mL | |
| 5 mM | 1.0743 mL | 5.3717 mL | 10.7434 mL | |
| 10 mM | 0.5372 mL | 2.6859 mL | 5.3717 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT04389281 | Recruiting | Combination Product: X-PACT | Advanced Solid Tumor Cancer | Immunolight, LLC | December 8, 2021 | Phase 1 |
| NCT00005092 | Completed | Drug: Psoralen Drug: Thiotepa |
Leukemia Lymphoma |
M.D. Anderson Cancer Center | May 28, 1999 | Phase 1 |
| NCT01526213 | Completed | Drug: Fexofenadine | Food-drug Interaction | University of North Carolina, Chapel Hill |
September 2009 | Not Applicable |
|
|
|