| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| 5g | |||
| Other Sizes |
| 靶点 |
Pyrimethamine targets the dihydrofolate reductase (DHFR) of malarial parasites (Plasmodium spp.), inhibiting the conversion of dihydrofolate to tetrahydrofolate (a critical cofactor for DNA synthesis). [1]
- Pyrimethamine exerts its anti-Toxoplasma effect by specifically inhibiting the DHFR of Toxoplasma gondii (TgDHFR), which has higher affinity for the drug than mammalian DHFR. [2] |
|---|---|
| 体外研究 (In Vitro) |
氟康唑 (FLZ) 与乙胺嘧啶(吡美西丹;4 nM–4 μM;24 小时;LLC-MK2 细胞与弓形虫)联合使用可抑制 FLZ 浓度为 0、0.05、0.1、0.5、1.0 和 3.0 的弓形虫活性μM,IC50 值分别为 0.23、0.19、0.23、0.34、0.14 和 0.19 μM[1]。
在疟原虫(种类未明确,推测为恶性疟原虫或间日疟原虫)体外培养体系中,用乙胺嘧啶(浓度范围:0.1-1 μM)处理后,寄生虫核分裂显著受阻。光镜和电镜观察显示:(1)裂殖体发育停滞,无法形成成熟裂殖子;(2)处理24小时后,60%-70%的寄生虫出现核固缩(凋亡样异常);(3)0.5 μM浓度下,寄生虫DNA合成(通过[3H]-胸腺嘧啶掺入法检测)较未处理对照组减少50%-60% [1] |
| 体内研究 (In Vivo) |
氟康唑、磺胺嘧啶与乙胺嘧啶(吡美西丹;1 mg/kg;ig;每天,持续 10 天;带有弓形虫异种移植物的雌性 CF1 小鼠)的组合可增强对死亡的保护[1]。
1968年的疟原虫核分裂体外研究未描述乙胺嘧啶的体内动物实验或药效数据 [1] - 在小鼠急性弓形虫病模型(6-8周龄Swiss Webster小鼠)中:小鼠腹腔注射1×10⁴个弓形虫RH株速殖子进行感染,乙胺嘧啶分为三组测试:(1)乙胺嘧啶单药组(10 mg/kg/天,灌胃);(2)乙胺嘧啶+磺胺嘧啶组(10 mg/kg + 100 mg/kg/天,灌胃);(3)乙胺嘧啶+磺胺嘧啶+氟康唑组(10 mg/kg + 100 mg/kg + 20 mg/kg/天,灌胃)。感染后24小时开始治疗,持续7天。结果:(1)乙胺嘧啶单药将死亡率从100%(未处理对照组)降至40%,脑速殖子载量(实时PCR检测)降低30%;(2)双药联合组(乙胺嘧啶+磺胺嘧啶)将死亡率降至20%,速殖子载量降低60%;(3)三药联合组进一步将死亡率降至10%,速殖子载量降低80%,证实协同疗效 [2] |
| 细胞实验 |
细胞活力测定[1]
细胞类型:含有弓形虫的 LLC-MK2 细胞 测试浓度: 4 nM-4 μM 孵化持续时间:24小时 实验结果:抑制弓形虫活性并降低寄生虫增殖指数。 疟原虫核分裂检测:疟原虫在含5%红细胞压积的RPMI 1640培养基中培养(补充10%人血清和25 mM HEPES)。加入浓度为0.1、0.5、1 μM的乙胺嘧啶,在37°C、5% CO₂环境中孵育。处理后12、24、48小时,收集100 μL培养物,用吉姆萨染液染色30分钟,在光镜下(1000×放大倍数)观察。每样本计数100个含虫红细胞中的正常裂殖体(核分裂完整)和异常寄生虫(核固缩)数量,通过异常寄生虫百分比评估药物作用 [1] |
| 动物实验 |
Animal/Disease Models: Female CF1 mice (18-22 g ; 4-6 week of age) with T. gondii xenograft[1]
Doses: po (oral gavage); daily, for 10 days Route of Administration: 1 mg/kg; 10 mg/kg (Fluconazole ), 40 mg/kg (Sulfadiazine) Experimental Results: Increased mouse survival compared to treatment with SDZ/PYR alone. Acute Toxoplasmosis Murine Model Protocol: (1) Mouse preparation: Female Swiss Webster mice (6-8 weeks old, 20-25 g) were acclimated for 7 days before infection. (2) Infection: Mice were intraperitoneally injected with 1×10⁴ T. gondii RH strain tachyzoites (suspended in 0.2 mL sterile PBS). (3) Drug preparation: Pyrimethamine was dissolved in 0.5% carboxymethyl cellulose (CMC) in sterile water; sulfadiazine and fluconazole were dissolved in the same solvent. (4) Treatment groups (n=10 mice/group): - Untreated control: 0.5% CMC (oral gavage, once daily); - Pyrimethamine alone: 10 mg/kg (oral gavage, once daily); - Pyrimethamine + sulfadiazine: 10 mg/kg + 100 mg/kg (oral gavage, once daily); - Pyrimethamine + sulfadiazine + fluconazole: 10 mg/kg + 100 mg/kg + 20 mg/kg (oral gavage, once daily). (5) Monitoring: Mice were monitored daily for mortality and clinical signs (e.g., lethargy, weight loss) for 21 days post-infection. At day 7 post-treatment, 3 mice per group were euthanized, and brain tissues were collected for tachyzoite load quantification via real-time PCR [2] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Well absorbed with peak levels occurring between 2 to 6 hours following administration The concentration of chloroquine, dapsone and pyrimethamine in plasma and milk were measured following the coadministration of a single dose of chloroquine and Maloprim to lactating women. The milk to plasma area under the concentration-time curve (AUC) ratio ranged from 1.96 to 4.26 for chloroquine, 0.22 to 0.45 for dapsone and 0.46 to 0.66 for pyrimethamine. Assuming a daily milk ingestion of 1 l by the infant, the maximum percentage of the maternal dose for chloroquine, dapsone and pyrimethamine in milk was 4.2%, 14.3% and 45.6%, respectively, over a 9 day period. Pyrimethamine is excreted into milk. It is estimated that approximately 3-4 mg of the drug would be ingested by a nursing infant over the first 48-hour period following administration of a single 75-mg oral dose to the mother. In this study, the kinetics of pyrimethamine elimination via the urine was investigated. The experiments were carried out on six healthy male volunteers aged 23-32 years. The drug was administered orally (p.o.) in a single dose at three different concentrations i.e.: 50, 75 and 100 mg. The concentration of the drug in the urine was determined via the modified method of Bonini et al. and Garber et al. It was found that 13.4 +/- 1.3% of the dose eliminated via the urine was in unchanged form. The process of pyrimethamine elimination may be described according to an open kinetic two-compartmental model: the formula showing the course of pyrimethamine elimination over time has been given. Several examples of the quantitative exposure test have been proposed, which allow the calculation of the drug dose absorbed and thus the degree of toxicity to be determined. This test can also be useful in a controlled clinical setting. A pharmacokinetic study of pyrimethamine was carried out in 4- (103-115 g) and 12-week-old (260-280 g) white male Wistar rats fed a standard diet containing 24% protein, and a low-protein diet containing 8% protein. After intragastric administration of the drug in a single dose of 40 mg/kg body weight, the concentrations of pyrimethamine in the blood were determined at different time points from 15 min to 20 hours post-dose. On the basis of the results obtained, a number of parameters characterizing the course of absorption and elimination of the drug from the blood were calculated. The majority of parameters were dependent on both age and type of diet. The greatest bioavailability was observed in the 4-week-old rats: for the animals fed the low-protein diet, the area under the concentration-time curve (AUC) amounted to 593.0 and for those on the standard diet the AUC was 503.1. In the older rats, this parameter was 339.3 and 228.1 respectively. The k(e) values were lower in the younger rats (i.e. 0.0121 hr(-1) and 0.0135 h(-1)) than in the older animals (i.e. 0.0164 h(-1) and 0.0193 hr(-1) respectively). The elimination half-life (t1/2) was higher in the 4-week-old rats (i.e. 57.1 hr; 8% protein, and 51.2 hr; 24% protein) than in the 12-week-old animals (i.e. 42.4 hr; 8% protein, and 36.0 hr; 24% protein). For more Absorption, Distribution and Excretion (Complete) data for Pyrimethamine (10 total), please visit the HSDB record page. Metabolism / Metabolites Hepatic Pyrimethamine is metabolized to several unidentified metabolites. About 5% of a dose of sulfadoxine is present in plasma as an acetylated metabolite and about 2-3% is present as the glucuronide. Hepatic Half Life: 96 hours Biological Half-Life 96 hours Pyrimethamine reportedly has an average plasma half-life of 111 hours (range: 54-148 hours). The plasma half-life of sulfadoxine reportedly averages 169 hours (range: 100-231 hours). To determine pyrimethamine levels in sera, cerebrospinal fluid, and ventricular fluid in infants, specimens were examined from 37 infants, ages 10 days to 1.5 yr, receiving pyrimethamine 1 mg/kg of body weight daily for 2 months followed by the same dosage each Monday, Wednesday, and Friday for treatment of suspect or proven congenital toxoplasmosis. The pyrimethamine half-life obtained from serum of 9 babies was 64 hr, which was significantly different than for 2 infants taking phenobarbital (33 hr). A pharmacokinetic study of pyrimethamine was carried out in 4- (103-115 g) and 12-week-old (260-280 g) white male Wistar rats fed a standard diet containing 24% protein, and a low-protein diet containing 8% protein. After intragastric administration of the drug in a single dose of 40 mg/kg body weight, the concentrations of pyrimethamine in the blood were determined at different time points from 15 min to 20 hours post-dose...The elimination half-life (t1/2) was higher in the 4-week-old rats (i.e. 57.1 hr; 8% protein, and 51.2 hr; 24% protein) than in the 12-week-old animals (i.e. 42.4 hr; 8% protein, and 36.0 hr; 24% protein). |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
Pyrimethamine inhibits the dihydrofolate reductase of plasmodia and thereby blocks the biosynthesis of purines and pyrimidines, which are essential for DNA synthesis and cell multiplication. This leads to failure of nuclear division at the time of schizont formation in erythrocytes and liver. Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation No adverse reactions in breastfed infants have been reported and it is acceptable in nursing mothers. In HIV-infected women, elevated viral HIV loads in milk were decreased after treatment with chloroquine to a greater extent than other women who were treated with the combination of sulfadoxine and pyrimethamine. It has been suggested that maternal pyrimethamine clearance might be increased during lactation, but data are insufficient to make a definitive conclusion. ◉ Effects in Breastfed Infants Administration of pyrimethamine to mothers of 26 predominantly breastfed infants 2 to 6 months old who were infected with malaria was curative in the infants.] The regimen consisted of 75 mg followed by a subsequent dose of 50 to 75 mg 4 to 7 days later. The efficacy apparently is related to breastfeeding habits, because infants in another tribal group who breastfed their infants less extensively were not protected. No adverse effects were reported in these infants. A case report indicates that a maternal dose of 75 mg orally followed by 25 mg weekly cured malaria in her breastfed infant and protected her infant against becoming infected with malaria for 6 months. After the mother missed taking her dose for 2 weeks, the infant developed symptoms of malaria. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding 87% Interactions Although the clinical importance is unclear, mild hepatotoxicity has been reported in some patients receiving pyrimethamine and lorazepam concomitantly. Although the clinical importance is unclear, p-aminobenzoic acid (PABA) reportedly interferes with the action of pyrimethamine and probably should not be used in patients receiving pyrimethamine. An increased incidence and severity of adverse effects has been reported when chloroquine was used concomitantly with the fixed combination of sulfadoxine and pyrimethamine compared with use of the fixed combination alone. Sulfadoxine and pyrimethamine is compatible with quinine and with other anti-infectives. Concomitant use of pyrimethamine or sulfadoxine and pyrimethamine with other antifolate agents (e.g., sulfonamides, co-trimoxazole, trimethoprim) is not recommended since such use may increase the risk of bone marrow suppression. If signs of folate deficiency develop, pyrimethamine or sulfadoxine and pyrimethamine should be discontinued and leucovorin administered (if necessary) until normal hematopoiesis is restored. For more Interactions (Complete) data for Pyrimethamine (6 total), please visit the HSDB record page. Non-Human Toxicity Values LD50 Rat intraperitoneal 70 mg/kg LD50 Mouse intraperitoneal 74 mg/kg LD50 Mouse oral 92 mg/kg In the 2013 murine study: Pyrimethamine (10 mg/kg/day, oral) caused mild, transient weight loss (5%-8%) in mice during the first 3 days of treatment, which recovered by day 7. No significant changes in serum alanine transaminase (ALT), aspartate transaminase (AST), or creatinine levels were observed compared to the untreated control group. No severe toxic effects (e.g., liver necrosis, renal damage) were detected in histological examination of liver and kidney tissues collected at day 7 post-treatment [2] |
| 参考文献 |
[1]. Aikawa M, et, al. Studies on nuclear division of a malarial parasite under pyrimethamine treatment. J Cell Biol. 1968 Dec;39(3):749-54.
[2]. Martins-Duarte ÉS, et, al. Toxoplasma gondii: the effect of fluconazole combined with sulfadiazine and pyrimethamine against acute toxoplasmosis in murine model. Exp Parasitol. 2013 Mar;133(3):294-9. |
| 其他信息 |
Therapeutic Uses
Although pyrimethamine has been used alone for suppression or chemoprophylaxis of malaria in travelers, the drug is no longer recommended by the US Centers for Disease Control and Prevention (CDC) or other experts for prevention of malaria. The manufacturer states that pyrimethamine should only be used for suppression or chemoprophylaxis of malaria caused by Plasmodium known to be susceptible to the drug. However, resistance to pyrimethamine is prevalent worldwide and the drug alone is not a suitable chemoprophylaxis regimen for travelers to most areas of the world. /Included in US product labeling/ Pyrimethamine is used in conjunction with sulfadiazine or, alternatively, clindamycin, atovaquone, or azithromycin for the treatment of toxoplasmosis caused by Toxoplasma gondii. /Included in US product labeling/ Oral or parenteral leucovorin is used with pyrimethamine in these regimens to prevent pyrimethamine-induced adverse hematologic effects. /Included in US product labeling/ Although co-trimoxazole generally is considered the drug of choice for the treatment of GI infections caused by Isospora belli, pyrimethamine has been used for the treatment of isosporiasis in some patients (e.g., HIV-infected patients) when co-trimoxazole was contraindicated, including those with sulfonamide sensitivity. /NOT included in US product labeling/ For more Therapeutic Uses (Complete) data for Pyrimethamine (16 total), please visit the HSDB record page. Drug Warnings High dosages of pyrimethamine may result in adverse nervous system effects including ataxia, tremors, seizures, and respiratory failure. Headache, light-headedness, insomnia, depression, malaise, fatigue, and irritability have been reported rarely with pyrimethamine. Reversible hyperesthesia has been reported rarely with sulfadoxine and pyrimethamine. Other adverse nervous system effects reported with sulfonamides or pyrimethamine include peripheral neuritis, hallucinations, tinnitus, vertigo, muscle weakness, nervousness, and polyneuritis. Sensitivity reactions, occasionally severe (e.g., Stevens-Johnson syndrome, toxic epidermal necrolysis, erythema multiforme, anaphylaxis) have been reported with pyrimethamine, especially when the drug was used with a sulfonamide. Severe, sometimes fatal, hypersensitivity reactions have occurred with the fixed-combination preparation of sulfadoxine and pyrimethamine. In most reported cases, fatalities resulted from severe cutaneous reactions, including erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis. Pulmonary hypersensitivity reactions and a fatal reaction involving the skin, liver, and kidneys also have been reported. Fatal hepatitis also has been reported with the fixed-combination drug. Severe reactions to sulfadoxine and pyrimethamine have occurred in travelers who received 2-9 doses of the drug for prophylaxis of malaria, but have not been reported to date following a single dose of the drug such as that used in the treatment of malaria. It is estimated that the incidence of severe cutaneous adverse reactions ranges from 1/8000 to 1/5000 and that the incidence of fatal cutaneous reactions ranges from 1/25,000 to 1/11,000 in US travelers receiving chemoprophylaxis with sulfadoxine and pyrimethamine. Anorexia, abdominal cramps, diarrhea, and vomiting may occur with high dosages of pyrimethamine. Anorexia and vomiting may be minimized by reducing dosage of pyrimethamine or by administering the drug with meals. Atrophic glossitis or gastritis also has been reported with high dosages of pyrimethamine. Other adverse GI effects reported with sulfonamides or with pyrimethamine include stomatitis, nausea, abdominal pain, and feeling of fullness. For more Drug Warnings (Complete) data for Pyrimethamine (21 total), please visit the HSDB record page. Pharmacodynamics Pyrimethamine is an antiparasitic compound commonly used as an adjunct in the treatment of uncomplicated, chloroquine resistant, P. falciparum malaria. Pyrimethamine is a folic acid antagonist and the rationale for its therapeutic action is based on the differential requirement between host and parasite for nucleic acid precursors involved in growth. This activity is highly selective against plasmodia and Toxoplasma gondii. Pyrimethamine possesses blood schizonticidal and some tissue schizonticidal activity against malaria parasites of humans. However, the 4-amino-quinoline compounds are more effective against the erythrocytic schizonts. It does not destroy gametocytes, but arrests sporogony in the mosquito. The action of pyrimethamine against Toxoplasma gondii is greatly enhanced when used in conjunction with sulfonamides. The 1968 study was one of the early investigations to elucidate the mechanism of Pyrimethamine against malaria: by inhibiting parasite DHFR, the drug blocks tetrahydrofolate-dependent DNA synthesis, leading to abnormal nuclear division and arrest of schizont maturation—this laid the foundation for its clinical use as an antimalarial agent [1] - In the 2013 study, the triple combination of Pyrimethamine + sulfadiazine + fluconazole showed superior efficacy against acute toxoplasmosis compared to Pyrimethamine alone or dual combinations. This is because fluconazole inhibits T. gondii ergosterol synthesis (a key component of parasite cell membranes), synergizing with the antifolate effect of Pyrimethamine to enhance parasite killing [2] |
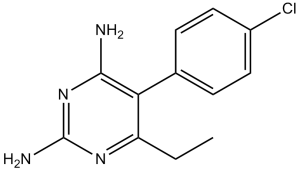
| 分子式 |
C12H13CLN4
|
|
|---|---|---|
| 分子量 |
248.71
|
|
| 精确质量 |
248.082
|
|
| CAS号 |
58-14-0
|
|
| 相关CAS号 |
Pyrimethamine-d3;1189936-99-9
|
|
| PubChem CID |
4993
|
|
| 外观&性状 |
Crystals
White scored tablets contains 25 mg pyrimethamine /Daraprim/ |
|
| 密度 |
1.4±0.1 g/cm3
|
|
| 沸点 |
368.4±52.0 °C at 760 mmHg
|
|
| 熔点 |
233-234°C
|
|
| 闪点 |
176.6±30.7 °C
|
|
| 蒸汽压 |
0.0±0.8 mmHg at 25°C
|
|
| 折射率 |
1.667
|
|
| LogP |
1.03
|
|
| tPSA |
77.82
|
|
| 氢键供体(HBD)数目 |
2
|
|
| 氢键受体(HBA)数目 |
4
|
|
| 可旋转键数目(RBC) |
2
|
|
| 重原子数目 |
17
|
|
| 分子复杂度/Complexity |
243
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
ClC1C([H])=C([H])C(=C([H])C=1[H])C1C(N([H])[H])=NC(N([H])[H])=NC=1C([H])([H])C([H])([H])[H]
|
|
| InChi Key |
WKSAUQYGYAYLPV-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C12H13ClN4/c1-2-9-10(11(14)17-12(15)16-9)7-3-5-8(13)6-4-7/h3-6H,2H2,1H3,(H4,14,15,16,17)
|
|
| 化学名 |
5-(4-chlorophenyl)-6-ethylpyrimidine-2,4-diamine
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (10.05 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (10.05 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL 澄清 DMSO 储备液加入到 900 μL 玉米油中并混合均匀。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 4.0207 mL | 20.1037 mL | 40.2075 mL | |
| 5 mM | 0.8041 mL | 4.0207 mL | 8.0415 mL | |
| 10 mM | 0.4021 mL | 2.0104 mL | 4.0207 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT05678348 | Recruiting | Drug: Pyrimethamine | Head and Neck Cancer Cancer of the Head and Neck |
Washington University School of Medicine |
August 3, 2023 | Early Phase 1 |
| NCT05497063 | Not yet recruiting | Drug: G-COSPE® tablets | Bioequivalence | Emzor Pharmaceutical Industries Limited |
December 2022 | Phase 1 |
| NCT03057990 | Withdrawn | Drug: Pyrimethamine | Myelodysplastic Syndromes | Montefiore Medical Center | September 11, 2019 | Phase 1 |
| NCT01102686 | Completed | Drug: Pyrimethamine Drug: Leucovorin |
Gangliosidoses, GM2 Sandhoff Disease |
The Hospital for Sick Children | August 2009 | Phase 1 Phase 2 |