| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 10g |
|
||
| Other Sizes |
|
| 靶点 |
Angiotensin-converting enzyme (ACE); Quinapril HCl (CI-906) inhibited human plasma ACE with an IC50 value of approximately 1.9 nM, and its active metabolite quinaprilat inhibited human plasma ACE with an IC50 value of approximately 0.16 nM [2]
|
|---|---|
| 体外研究 (In Vitro) |
血管紧张素转换酶抑制剂(ACE 抑制剂)如喹那普利 (HCl) (CI-906) 用于治疗充血性心力衰竭和高血压。吸收后,喹那普利迅速去酯化形成喹那普利,一种血管紧张素转换酶 (ACE) 的有效抑制剂 [1][2]。
Quinapril HCl (CI-906) 在体外对血管紧张素转换酶(ACE)表现出剂量依赖性抑制活性。在人血浆ACE实验中,Quinapril HCl (CI-906) 的抑制作用弱于其活性代谢产物喹那普利拉,两者IC50值分别约为1.9 nM和0.16 nM [2] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Absorption (bioavailability) of quinapril is 60%; time to peak serum concn is 2 hr; half-life (elimination) is 2 hr; protein binding is 97%; metabolism is in the liver. /from table/ /Salt not specified/ Quinapril is rapidly absorbed (peak concns are achieved in 1 hr, but the peak may be delayed after food), & its rate but not extent of oral absorption (60%) may be reduced by food. Quinapril is metabolized to quinaprilat & to other minor metabolites, & quinaprilat is excreted in the urine (61%) & the feces (37%). Peak concns of quinaprilat in plasma are achieved in about 2 hr. Conversion of quinapril to quinaprilat is reduced in patients with diminished liver function. The initial half-life of quinaprilat is about 2 hr; a prolonged terminal half-life of about 25 hr may be due to high-affinity binding of the drug to tissue ACE. /Salt not specified/ Metabolism / Metabolites Cleavage of the ester moiety by hepatic esterases transforms quinapril hydrochloride, a prodrug, into quinaprilat, an ACE inhibitor that in vitro is about as potent as benazeprilat. ... Quinapril is metabolized to quinaprilat & to other minor metabolites ... . Biological Half-Life The initial half-life of /the metabolite/ quinaprilat is about 2 hr; a prolonged terminal half-life of about 25 hr may be due to high-affinity binding of the drug to tissue ACE. /Salt not specified/ Absorption: After oral administration of Quinapril HCl (CI-906) (10–80 mg) to healthy volunteers, the oral bioavailability of quinapril (parent drug) was approximately 60%, while the bioavailability of its active metabolite quinaprilat was approximately 25% (due to first-pass metabolism). Food intake reduced the peak plasma concentration (Cmax) of quinaprilat by ~30% but did not significantly affect the area under the plasma concentration-time curve (AUC) [1][2] - Distribution: The volume of distribution (Vd) of quinaprilat (active metabolite) in healthy volunteers was approximately 0.2 L/kg. Quinapril HCl (CI-906) and quinaprilat did not penetrate the blood-brain barrier significantly [2] - Metabolism: Quinapril HCl (CI-906) was rapidly metabolized in the liver via ester hydrolysis to form its active metabolite quinaprilat (the main pharmacologically active form). A small portion of quinapril was also metabolized to inactive conjugates (e.g., glucuronides) [1][2] - Excretion: Quinaprilat was excreted mainly via the kidneys and feces. In healthy volunteers, approximately 39% of the administered dose was excreted as quinaprilat in urine within 24 hours, and ~15% was excreted in feces. The elimination half-life (t1/2) of quinaprilat was approximately 2.5–3 hours in healthy volunteers; in patients with severe renal impairment (creatinine clearance <30 mL/min), the t1/2 of quinaprilat prolonged to ~10 hours [1][2] |
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation Because of the low levels of quinapril in breastmilk, amounts ingested by the infant are small and would not be expected to cause any adverse effects in breastfed infants. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Interactions Hyperkalemia may occur with potassium supplements, potassium-sparing agents, & NSAIDs. /ACE inhibitors; from table/ /Salt not specified/ ... ACE inhibitors enhance the efficacy of diuretic drugs. This means that even very small doses of diuretics may substantially improve the antihypertensive efficacy of ACE inhibitors; & on the other end of the spectrum, the use of high doses of diuretics together with angiotensin converting enzyme inhibitors may lead to excessive reduction in blood pressure & to /sodium ion/ loss in some patients. /ACE inhibitors/ /Salt not specified/ Oligopeptidic drugs such as beta-lactams & angiotensin-converting enzyme inhibitors share the same carriers in humans & animals, which results in possible pharmacokinetic interactions. To model such interactions, the effects of quinapril on cephalexin pharmacokinetics were investigated in rats. Blood cephalexin concns were measured by liquid chromatography, & the data were analyzed by a noncompartmental method & by fitting a bicompartmental model by a nonlinear mixed-effect modeling approach. 5 groups of 8 rats were examined. In the first 3 groups, cephalexin elimination kinetics after intra-arterial admin alone or in combination with quinapril given by the parenteral or the oral route were studied, & the occurrence of a pharmacokinetic interaction was not revealed. The absence of an effect of quinapril on cephalexin elimination after parenteral admin might be explained either by the higher affinity of cephalexin for the renal anionic transport system than that of quinapril or by the much higher concns of cephalexin than those of quinapril. In the last 2 groups, cephalexin was administered by the oral route alone or in combination with quinapril. The mean area under the concn-time curve (AUC) for cephalexin was increased by ca. 30% by coadmin of quinapril (40.1 versus 31.4 mg.hr/liter; P=0.04). The mean elimination clearance of cephalexin was significantly decreased by quinapril, from 0.81 to 0.64 liter/hr/kg of body weight (P<0.05), probably by competitive inhibition of cephalexin secretion at the tubular level. The mean absorption rate constant of cephalexin was significantly lowered by quinapril (from 0.249 to 0.177 hr-1; P<0.01), without modification of the extent of absorption (89%). This pharmacokinetic interaction could be explained by competitive inhibition of cephalexin active transport by quinapril at the intestinal level. /Salt not specified/ Concurrent use /of other hypotension-producing medications/ with ACE inhibitors may produce additive hypotensive effects. /ACE inhibitors/ /Salt not specified/ For more Interactions (Complete) data for QUINAPRIL HYDROCHLORIDE (16 total), please visit the HSDB record page. Non-Human Toxicity Values LD50 in male, female mice, rats (mg/kg): 1739, 1840, 4280, 3541 orally; 504, 523, 158, 107 i.v. /Salt not specified/ Plasma protein binding: Quinapril HCl (CI-906) had a plasma protein binding rate of approximately 97%, and its active metabolite quinaprilat had a plasma protein binding rate of approximately 95% [2] - Adverse effects: The most common adverse effects of Quinapril HCl (CI-906) in clinical studies included hypotension (especially in patients receiving diuretics concomitantly), dry cough, headache, dizziness, and fatigue. Rare adverse effects included angioedema, hyperkalemia, and renal dysfunction (more common in patients with pre-existing renal impairment or heart failure) [1][2] - Drug-drug interactions: Concomitant use of Quinapril HCl (CI-906) with diuretics (e.g., furosemide) increased the risk of hypotension. Concomitant use with non-steroidal anti-inflammatory drugs (NSAIDs, e.g., indomethacin) reduced the antihypertensive effect of Quinapril HCl (CI-906) and may increase the risk of renal impairment. Concomitant use with potassium-sparing diuretics (e.g., spironolactone) or potassium supplements increased the risk of hyperkalemia [1][2] |
| 参考文献 |
|
| 其他信息 |
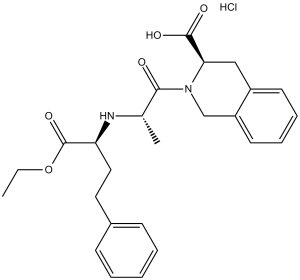
Quinapril hydrochloride is a hydrochloride resulting from the reaction of equimolar amounts of quinapril and hydrogen chloride. A prodrug for quinaprilat hydrochloride (by hydrolysis of the ethyl ester to the corresponding carboxylic acid), it is used as an angiotensin-converting enzyme inhibitor (ACE inhibitor) for the treatment of hypertension and congestive heart failure. It has a role as an antihypertensive agent and an EC 3.4.15.1 (peptidyl-dipeptidase A) inhibitor. It contains a quinapril(1+).
Quinapril Hydrochloride is the hydrochloride salt form of quinapril, a prodrug and non-sulfhydryl angiotensin converting enzyme (ACE) inhibitor with antihypertensive activity. Quinapril is hydrolized into its active form quinaprilat, which binds to and inhibits ACE, thereby blocking the conversion of angiotensin I to angiotensin II. This abolishes the potent vasoconstrictive actions of angiotensin II and leads to vasodilatation. Quinapril also causes a decrease in angiotensin II-induced aldosterone secretion by the adrenal cortex, thereby promoting diuresis and natriuresis, and increases bradykinin levels. A tetrahydroisoquinoline derivative and ANGIOTENSIN CONVERTING ENZYME inhibitor that is used in the treatment of HYPERTENSION and HEART FAILURE. See also: Quinapril (has active moiety); Quinaprilat (has active moiety); Hydrochlorothiazide; quinapril hydrochloride (component of). Mechanism of Action Block formation of angiotensin II, promoting vasodilation & decreased aldosterone; also increased bradykinin & vasodilatory prostaglandins. /ACE Inhibitors; from table/ /Salt not specified/ Quinapril is deesterified to the principal metabolite, quinaprilat, which is an inhibitor of ACE activity in human subjects and animals. ACE is a peptidyl dipeptidase that catalyzes the conversion of angiotensin I to the vasoconstrictor, angiotensin II. The effect of quinapril in hypertension and in congestive heart failure (CHF) appears to result primarily from the inhibition of circulating and tissue ACE activity, thereby reducing angiotensin II formation. Quinapril inhibits the elevation in blood pressure caused by iv administered angiotensin I, but has no effect on the pressor response to angiotensin II, norepinephrine or epinephrine. Angiotensin II also stimulates the secretion of aldosterone from the adrenal cortex, thereby facilitating renal sodium and fluid reabsorption. Reduced aldosterone secretion by quinapril may result in a small incr in serum potassium. In controlled hypertension trials, treatment with ACCUPRIL alone resulted in mean increases in potassium of 0.07 mmol/L ... . Removal of angiotensin II negative feedback on renin secretion leads to increased plasma renin activity (PRA). /Salt not specified/ Therapeutic Uses ... /Quinapril/ has proven to be very useful for the treatment of hypertension ... . /Salt not specified/ The angiotensin converting enzyme (ACE) inhibitors appear to confer a special advantage in the treatment of patients with diabetes, slowing the development of diabetic glomerulopathy. They also have been shown to be effective in slowing the progression of other forms of chronic renal disease, such as glomerulosclerosis, & many of these patients also have hypertension. An ACE inhibitor is probably the preferred initial agent in the treatment of hypertensive patients with left ventricular hypertrophy. Patients with hypertension & ischemic heart disease are candidates for treatment with ACE inhibitors; this includes treatment in the immediate post-myocardial infarction period which has been shown to lead to improved ventricular function & reduced morbidity & mortality. /ACE inhibitors/ /Salt not specified/ The combination of ... quinapril and hydrochlorothiazide is indicated in the treatment of hypertension. Fixed-dosage combinations generally are not recommended for initial therapy, but are utilized in maintenance therapy after the required dose is established in order to increase convenience, economy, and patient compliance. /Included in US product labeling/ /Salt not specified/ Angiotensin converting enzyme (ACE) inhibitor /Salt not specified/ For more Therapeutic Uses (Complete) data for QUINAPRIL HYDROCHLORIDE (6 total), please visit the HSDB record page. Drug Warnings Reduce dose ... in patients with serum creatinine > or =221 umol/L (2.5 mg/dL). /ACE Inhibitors; from table/ /Salt not specified/ May cause hyperkalemia in patients with renal impairment or in those receiving potassium-sparing agents. /ACE Inhibitors; from table/ /Salt not specified/ Can cause acute renal failure in patients with severe bilateral renal artery stenosis or severe stenosis in artery to solitary kidney. /ACE Inhibitors; from table/ /Salt not specified/ Conversion of quinapril to quinaprilat is reduced in patients with diminished liver function. /Salt not specified/ For more Drug Warnings (Complete) data for QUINAPRIL HYDROCHLORIDE (12 total), please visit the HSDB record page. Quinapril HCl (CI-906) is an oral, prodrug-type angiotensin-converting enzyme inhibitor (ACEI). It exerts its pharmacological effects by being metabolized to quinaprilat, which inhibits ACE, thereby reducing the conversion of angiotensin I to angiotensin II (a potent vasoconstrictor) and increasing bradykinin levels (a vasodilator) [1][2] - Therapeutic indications: Quinapril HCl (CI-906) is indicated for the treatment of essential hypertension (as monotherapy or in combination with other antihypertensive drugs) and congestive heart failure (as adjunctive therapy with diuretics and/or digoxin) [2] - In patients with hypertension, oral administration of Quinapril HCl (CI-906) (10–40 mg once daily) resulted in a sustained reduction in systolic and diastolic blood pressure, with peak antihypertensive effects occurring 2–4 hours after administration and duration of action lasting >24 hours [1][2] |
| 分子式 |
C25H30N2O5.HCL
|
|
|---|---|---|
| 分子量 |
474.98
|
|
| 精确质量 |
474.192
|
|
| CAS号 |
82586-55-8
|
|
| 相关CAS号 |
Quinapril-d5;1279029-79-6
|
|
| PubChem CID |
54891
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 沸点 |
662ºC at 760 mmHg
|
|
| 熔点 |
120-130ºC
|
|
| 闪点 |
354.1ºC
|
|
| LogP |
3.697
|
|
| tPSA |
95.94
|
|
| 氢键供体(HBD)数目 |
3
|
|
| 氢键受体(HBA)数目 |
6
|
|
| 可旋转键数目(RBC) |
10
|
|
| 重原子数目 |
33
|
|
| 分子复杂度/Complexity |
648
|
|
| 定义原子立体中心数目 |
3
|
|
| SMILES |
CCOC(=O)[C@H](CCC1=CC=CC=C1)N[C@@H](C)C(=O)N2CC3=CC=CC=C3C[C@H]2C(=O)O.Cl
|
|
| InChi Key |
IBBLRJGOOANPTQ-JKVLGAQCSA-N
|
|
| InChi Code |
InChI=1S/C25H30N2O5.ClH/c1-3-32-25(31)21(14-13-18-9-5-4-6-10-18)26-17(2)23(28)27-16-20-12-8-7-11-19(20)15-22(27)24(29)30;/h4-12,17,21-22,26H,3,13-16H2,1-2H3,(H,29,30);1H/t17-,21-,22-;/m0./s1
|
|
| 化学名 |
(3S)-2-[(2S)-2-[[(2S)-1-ethoxy-1-oxo-4-phenylbutan-2-yl]amino]propanoyl]-3,4-dihydro-1H-isoquinoline-3-carboxylic acid;hydrochloride
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (5.26 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (5.26 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (5.26 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 100 mg/mL (210.54 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶 (<60°C). 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.1054 mL | 10.5268 mL | 21.0535 mL | |
| 5 mM | 0.4211 mL | 2.1054 mL | 4.2107 mL | |
| 10 mM | 0.2105 mL | 1.0527 mL | 2.1054 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。