| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 50mg |
|
||
| 100mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| 10g | |||
| Other Sizes |
| 靶点 |
Angiotensin-converting enzyme (ACE); the IC50 value for inhibiting rabbit small intestinal brush border membrane ACE was 0.02 μM [2]
|
|---|---|
| 体外研究 (In Vitro) |
血管紧张素转换酶 (ACE) 抑制剂雷米普利 (HOE-498) 的 IC50 为 5 nM[1]。然而,在表达 S1270A ACE 突变体的内皮细胞或 ACE 缺陷细胞中,雷米普利 (HOE-498) 无法激活 JNK 或促进 c-Jun 的核积累。相反,它会增加培养的内皮细胞中 ACE 相关 CK2 的活性和 ACE Ser1270 的磷酸化。长期使用雷米普利会增加小鼠体内肺和人内皮细胞原代培养物中 ACE 的表达,这种现象可以通过用 JNK 抑制剂 SP600125 预处理来避免[2]。
Ramipril (HOE-498)对从兔小肠刷状缘膜中分离的血管紧张素转换酶(ACE)具有抑制活性。在以Hippuryl-His-Leu为底物的酶活性实验中,Ramipril (HOE-498)呈剂量依赖性地降低ACE介导的底物水解反应。实验测定Ramipril (HOE-498)对兔小肠刷状缘膜ACE的半数最大抑制浓度(IC50)为0.02 μM [2] |
| 体内研究 (In Vivo) |
与体外细胞凋亡效应相反,与其他 ACE 抑制剂相比,以在体外 HUVEC 中具有相似降压作用的剂量对大鼠长期体内给予雷米普利 (HOE-498),可显着降低 LPS 诱导的细胞凋亡率 [3 [4]。
|
| 酶活实验 |
兔小肠刷状缘膜的制备:取出兔小肠,刮取肠黏膜。将黏膜组织在适宜缓冲液中匀浆,随后进行差速离心(包括低速离心去除组织碎片、高速离心沉淀刷状缘膜)。将得到的膜沉淀重悬于缓冲液中,获得含ACE的酶源。
- ACE活性测定流程:反应体系包含制备好的兔小肠刷状缘膜酶液、底物Hippuryl-His-Leu(溶于缓冲液)以及不同浓度的Ramipril (HOE-498)。体系在37℃下孵育特定时间后,加入三氯乙酸终止反应。随后离心去除沉淀的蛋白质,测定上清液在228 nm处的吸光度,以确定ACE水解底物生成的马尿酸量。根据吸光度值计算ACE活性,并计算各浓度Ramipril (HOE-498)对ACE的抑制率,通过量效曲线得出IC50值 [2] |
| 动物实验 |
Dissolved in distilled water by using gum arabic (10% w/v); 0.03-10 mg/kg; Oral gavage
Male spontaneously hypertensive rats |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
The extent of absorption is at least 50-60%.. Food decreases the rate of absorption from the GI tract without affecting the extent of absorption. The absolute bioavailabilities of ramipril and ramiprilat were 28% and 44%, respectively, when oral administration was compared to intravenous administration. The serum concentration of ramiprilat was unchanged when capsules were opened and the contents dissolved in water, dissolved in apple juice, or suspended in apple sauce. Following oral administration, about 60% of the dose is eliminated in the urine as unchanged ramipril (<2%) and its metabolites. About 40% of the dose is found in the feces, representing both unabsorbed drug and drugs and metabolites eliminated via biliary excretion. The urinary excretion of ramipril may be reduced in patients with impaired renal function. The renal clearance of ramipril and ramiprilat was reported to be 7.2 and 77.4 mL/min/1.73m 2 . The mean renal clearance of ramipril and ramiprilat is reported to be 10.7 and 126.8 mL/min in healthy elderly patients with normal renal function, additionally the Cmax of ramiprilat is approximately 20% higher in this population. While the pharmacokinetics of ramipril appear unaffected by reduced renal function, the plasma concentration and half-life of ramiprilat are increased. In patient's with hepatic failure the concentration of ramipril is initially increased while the tmax of ramiprilat is prolonged due to a reduced ability to metabolize the drug. However, steady state concentrations of ramiprilat are the same in hepatic failure as in healthy patients. /MILK/ Ingestion of a single 10 mg oral dose of ramipril resulted in undetectable amounts of ramipril and its metabolites in breast milk. Following oral administration of ramipril, peak plasma concentrations (Cmax) of ramipril are reached within 1 hour. The extent of absorption is at least 50% to 60%, and is not significantly influenced by the presence of food in the gastrointestinal tract, although the rate of absorption is reduced. Plasma concentrations of ramiprilat decline in a triphasic manner (initial rapid decline, apparent elimination phase, terminal elimination phase). The initial rapid decline, which represents distribution of the drug into a large peripheral compartment and subsequent binding to both plasma and tissue ACE, has a half-life of 2 to 4 hours. Because of its potent binding to ACE and slow dissociation from the enzyme, ramiprilat shows two elimination phases. The apparent elimination phase corresponds to the clearance of free ramiprilat and has a half-life of 9 to 18 hours. The terminal elimination phase has a prolonged half-life (>50 hours) and probably represents the binding/dissociation kinetics of the ramiprilat/ACE complex. It does not contribute to the accumulation of the drug. After multiple daily doses of ramipril 5 mg to 10 mg, the half-life of ramiprilat concentrations within the therapeutic range was 13 to 17 hours. /Ramiprilat/ Plasma concentrations of ramipril and ramiprilat increase with increased dose, but are not strictly dose-proportional. The 24-hour AUC for ramiprilat, however, is dose-proportional over the 2.5 mg to 20 mg dose range. The absolute bioavailabilities of ramipril and ramiprilat were 28% and 44%, respectively, when 5 mg of oral ramipril was compared with the same dose of ramipril given intravenously. For more Absorption, Distribution and Excretion (Complete) data for Ramipril (7 total), please visit the HSDB record page. Metabolism / Metabolites Hepatic metabolism accounts for 75% of total ramipril metabolism. 25% of hepatic metabolism produces the active metabolite ramiprilat via liver esterase enzymes. 100% of renal metabolism converts ramipril to ramiprilat. Other metabolites, diketopiperazine ester, the diketopiperazine acid, and the glucuronides of ramipril and ramiprilat, are inactive. Cleavage of the ester group (primarily in the liver) converts ramipril to its active diacid metabolite, ramiprilat. Peak plasma concentrations of ramiprilat are reached 2 to 4 hours after drug intake. The serum protein binding of ramipril is about 73% and that of ramiprilat about 56%; in vitro, these percentages are independent of concentration over the range of 0.01 ug/mL to 10 mcg/mL. After oral administration to dogs, ramipril is rapidly converted via de-esterification into ramiprilat. Bioavailability of ramiprilat after a dose of 0.25 mg/kg per day of ramipril is approximately 6.7%. Ramipril is a prodrug and has little pharmacologic activity until hydrolyzed in the liver to ramiprilat. Ramipril is almost completely metabolized to ramiprilat, which has about 6 times the angiotensin-converting enzyme (ACE) inhibitory activity of ramipril, and to the diketopiperazine ester, the diketopiperazine acid, and the glucuronides of ramipril and ramiprilat, all of which are inactive. Biological Half-Life Plasma concentrations of ramiprilat decline in a triphasic manner. Initial rapid decline represents distribution into tissues and has a half life of 2-4 hours. The half life of the apparent elimination phase is 9-18 hours, which is thought to represent clearance of free drug. The half-life of the terminal elimination phase is > 50 hours and thought to represent clearance of drug bound to ACE due to its slow dissociation. The half life of ramiprilat after multiple daily doses (MDDs) is dose-dependent, ranging from 13-17 hours with 5-10 mg MDDs to 27-36 hours for 2.5 mg MDDs. Plasma concentrations of ramiprilat /metabolite of ramipril/ decline in a triphasic manner (initial rapid decline, apparent elimination phase, terminal elimination phase). The initial rapid decline, which represents distribution of the drug into a large peripheral compartment and subsequent binding to both plasma and tissue ACE, has a half-life of 2 to 4 hours. Because of its potent binding to ACE and slow dissociation from the enzyme, ramiprilat shows two elimination phases. The apparent elimination phase corresponds to the clearance of free ramiprilat and has a half-life of 9 to 18 hours. The terminal elimination phase has a prolonged half-life (>50 hours) and probably represents the binding/dissociation kinetics of the ramiprilat/ACE complex. It does not contribute to the accumulation of the drug. After multiple daily doses of ramipril 5 mg to 10 mg, the half-life of ramiprilat concentrations within the therapeutic range was 13 to 17 hours. /Ramiprilat/ |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION AND USE: Ramipril is a prodrug and has little pharmacologic activity until hydrolyzed in the liver to ramiprilat. It is an angiotensin-converting enzyme (ACE) inhibitor indicated for the treatment of hypertension. It is also used for stable patients with demonstratable congestive heart failure within the first few days of sustaining acute myocardial infarction. HUMAN STUDIES: Rare ACE inhibitor-associated clinical syndrome manifested initially by cholestatic jaundice may occur. It may progress to fulminant hepatic necrosis and is potentially fatal. Patients receiving an ACE inhibitor, including ramipril, who develop jaundice or marked elevations in hepatic enzymes should discontinue the drug and receive appropriate monitoring. Sensitivity reactions, including anaphylactic reactions and angioedema (including laryngeal or tongue edema) are potentially fatal. Head and neck angioedema involving the tongue, glottis, or larynx may cause airway obstruction. If laryngeal stridor or angioedema of the face, tongue, or glottis occurs, ramipril should be discontinued and appropriate therapy (e.g., epinephrine) should be initiated immediately. Use of drugs that act on the renin-angiotensin system during the second and third trimesters of pregnancy reduces fetal renal function and increases fetal and neonatal morbidity and death. Resulting oligohydramnios can be associated with fetal lung hypoplasia and skeletal deformations. Potential neonatal adverse effects include skull hypoplasia, anuria, hypotension, renal failure, and death. When pregnancy is detected, discontinue ramipril as soon as possible. No mutagenic activity was detected in the unscheduled DNA synthesis in a human cell line. ANIMAL STUDIES: No evidence of a tumorigenic effect was found when ramipril was given by gavage to rats for up to 24 months at doses of up to 500 mg/kg/day or to mice for up to 18 months at doses of up to 1000 mg/kg/day. A study in rats with dosages as great as 500 mg/kg/day did not produce adverse effects on fertility. Kidney organogenesis and functional development continue well into the postnatal period in the rat. A within-litter design was used to characterize renal susceptibility to an ACE inhibitor during the third week of life in rats. There were no treatment-related effects in rats dosed on PND 21. Following dosing on PND 14, dose-related increases were noted in mean serum urea nitrogen and/or creatinine levels on PND 17, but these measures recovered by PND 28. The interim changes were accompanied by macroscopic and microscopic changes in the kidneys on PND 17, including tubular hypoplasia, renal papillary edema, cortical tubular dilatation, hydronephrosis (pelvic dilatation) and tubular basophilia; renal anatomic changes were still evident and more severe on PND 28, 14 days after dosing. No mutagenic activity was detected in the Ames test in bacteria, the micronucleus test in mice or a forward gene-mutation assay in a Chinese hamster ovary cell line. Hepatotoxicity Ramipril, like other ACE inhibitors, has been associated with a low rate of serum aminotransferase elevations ( Likelihood score: C (probable rare cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Because no information is available on the use of ramipril during breastfeeding, an alternate drug may be preferred, especially while nursing a newborn or preterm infant. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Protein binding of ramipril is about 73% and that of ramiprilat about 56%. Protein binding is independent of concentration over the range of 0.1μg/mL-10μg/mL Interactions In patients who are elderly, volume-depleted (including those on diuretic therapy), or with compromised renal function, co-administration of non-ateroidal anti-inflammatory drugs (NSAIDs), including selective cyclooxygenase-2 (COX-2) inhibitors, with angiotensin-converting enzyme (ACE) inhibitors, including ramipril, may result in deterioration of renal function, including possible acute renal failure. These effects are usually reversible. Monitor renal function periodically in patients receiving ramipril and NSAID therapy. The antihypertensive effect of ACE inhibitors, including ramipril, may be attenuated by NSAIDs. Increased serum lithium levels and symptoms of lithium toxicity have been reported in patients receiving ACE inhibitors during therapy with lithium; therefore, frequent monitoring of serum lithium levels is recommended. If a diuretic is also used, the risk of lithium toxicity may be increased. Coadministration of ramipril with other drugs that raise serum potassium levels may result in hyperkalemia. Monitor serum potassium in such patients. Patients on diuretics, especially those in whom diuretic therapy was recently instituted, may occasionally experience an excessive reduction of blood pressure after initiation of therapy with ramipril. The possibility of hypotensive effects with ramipril can be minimized by either decreasing or discontinuing the diuretic or increasing the salt intake prior to initiation of treatment with ramipril. If this is not possible, reduce the starting dose. |
| 参考文献 |
|
| 其他信息 |
Therapeutic Uses
Angiotensin-Converting Enzyme Inhibitors; Antihypertensive Agents /CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Ramipril is included in the database. Ramipril capsules are indicated for the treatment of hypertension, to lower blood pressure. Lowering blood pressure reduces the risk of fatal and nonfatal cardiovascular events, primarily strokes and myocardial infarctions. These benefits have been seen in controlled trials of antihypertensive drugs from a wide variety of pharmacologic classes including this drug. ... Ramipril capsules may be used alone or in combination with thiazide diuretics. /Included in US product label/ Ramipril capsules are indicated in stable patients who have demonstrated clinical signs of congestive heart failure within the first few days after sustaining acute myocardial infarction. Administration of ramipril capsules to such patients have been shown to decrease the risk of death (principally cardiovascular death) and to decrease the risks of failure-related hospitalization and progression to severe/resistant heart failure. /Included in US product label/ For more Therapeutic Uses (Complete) data for Ramipril (8 total), please visit the HSDB record page. Drug Warnings /BOXED WARNING/ When pregnancy is detected, discontinue ramipril as soon as possible. Drugs that act directly on the renin-angiotensin system can cause injury and death to the developing fetus. Use of drugs that act on the renin-angiotensin system during the second and third trimesters of pregnancy reduces fetal renal function and increases fetal and neonatal morbidity and death. Resulting oligohydramnios can be associated with fetal lung hypoplasia and skeletal deformations. Potential neonatal adverse effects include skull hypoplasia, anuria, hypotension, renal failure, and death. When pregnancy is detected, discontinue ramipril as soon as possible. These adverse outcomes are usually associated with use of these drugs in the second and third trimester of pregnancy. Most epidemiologic studies examining fetal abnormalities after exposure to antihypertensive use in the first trimester have not distinguished drugs affecting the renin-angiotensin system from other antihypertensive agents. Appropriate management of maternal hypertension during pregnancy is important to optimize outcomes for both mother and fetus. Angiotensin-converting enzyme (ACE) inhibitors can cause fetal and neonatal morbidity and mortality when used in pregnancy during the second and third trimesters. ACE inhibitors also increase the risk of major congenital malformations when administered during the first trimester of pregnancy. Discontinue as soon as possible when pregnancy is detected, unless continued use is considered lifesaving. Nearly all women can be transferred successfully to alternative therapy for the remainder of their pregnancy. /Angiotensin-converting enzyme (ACE) inhibitors/ Rare angiotensin-converting enzyme (ACE) inhibitor-associated clinical syndrome manifested initially by cholestatic jaundice may occur; may progress to fulminant hepatic necrosis and is potentially fatal. Patients receiving an ACE inhibitor, including ramipril, who develop jaundice or marked elevations in hepatic enzymes should discontinue the drug and receive appropriate monitoring. For more Drug Warnings (Complete) data for Ramipril (18 total), please visit the HSDB record page. Pharmacodynamics Ramipril is an ACE inhibitor similar to benazepril, fosinopril and quinapril. It is an inactive prodrug that is converted to ramiprilat in the liver, the main site of activation, and kidneys. Ramiprilat confers blood pressure lowing effects by antagonizing the effect of the RAAS. The RAAS is a homeostatic mechanism for regulating hemodynamics, water and electrolyte balance. During sympathetic stimulation or when renal blood pressure or blood flow is reduced, renin is released from the granular cells of the juxtaglomerular apparatus in the kidneys. In the blood stream, renin cleaves circulating angiotensinogen to ATI, which is subsequently cleaved to ATII by ACE. ATII increases blood pressure using a number of mechanisms. First, it stimulates the secretion of aldosterone from the adrenal cortex. Aldosterone travels to the distal convoluted tubule (DCT) and collecting tubule of nephrons where it increases sodium and water reabsorption by increasing the number of sodium channels and sodium-potassium ATPases on cell membranes. Second, ATII stimulates the secretion of vasopressin (also known as antidiuretic hormone or ADH) from the posterior pituitary gland. ADH stimulates further water reabsorption from the kidneys via insertion of aquaporin-2 channels on the apical surface of cells of the DCT and collecting tubules. Third, ATII increases blood pressure through direct arterial vasoconstriction. Stimulation of the Type 1 ATII receptor on vascular smooth muscle cells leads to a cascade of events resulting in myocyte contraction and vasoconstriction. In addition to these major effects, ATII induces the thirst response via stimulation of hypothalamic neurons. ACE inhibitors inhibit the rapid conversion of ATI to ATII and antagonize RAAS-induced increases in blood pressure. ACE (also known as kininase II) is also involved in the enzymatic deactivation of bradykinin, a vasodilator. Inhibiting the deactivation of bradykinin increases bradykinin levels and may sustain the effects of ramiprilat by causing increased vasodilation and decreased blood pressure. Ramipril (HOE-498) is a member of the angiotensin-converting enzyme inhibitor (ACEI) class, which exerts biological effects by inhibiting the activity of ACE [2] |
| 分子式 |
C23H32N2O5
|
|
|---|---|---|
| 分子量 |
416.51
|
|
| 精确质量 |
416.231
|
|
| CAS号 |
87333-19-5
|
|
| 相关CAS号 |
Ramipril-d5;1132661-86-9;Ramipril-d3;2673269-81-1
|
|
| PubChem CID |
5362129
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 密度 |
1.2±0.1 g/cm3
|
|
| 沸点 |
616.2±55.0 °C at 760 mmHg
|
|
| 熔点 |
106-108°C
|
|
| 闪点 |
326.4±31.5 °C
|
|
| 蒸汽压 |
0.0±1.9 mmHg at 25°C
|
|
| 折射率 |
1.556
|
|
| LogP |
3.41
|
|
| tPSA |
95.94
|
|
| 氢键供体(HBD)数目 |
2
|
|
| 氢键受体(HBA)数目 |
6
|
|
| 可旋转键数目(RBC) |
10
|
|
| 重原子数目 |
30
|
|
| 分子复杂度/Complexity |
619
|
|
| 定义原子立体中心数目 |
5
|
|
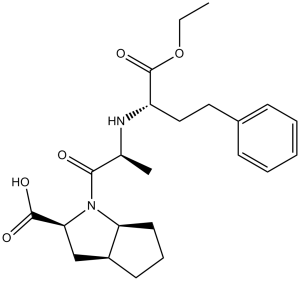
| SMILES |
CCOC(=O)[C@H](CCC1=CC=CC=C1)N[C@@H](C)C(=O)N2[C@H]3CCC[C@H]3C[C@H]2C(=O)O
|
|
| InChi Key |
HDACQVRGBOVJII-JBDAPHQKSA-N
|
|
| InChi Code |
InChI=1S/C23H32N2O5/c1-3-30-23(29)18(13-12-16-8-5-4-6-9-16)24-15(2)21(26)25-19-11-7-10-17(19)14-20(25)22(27)28/h4-6,8-9,15,17-20,24H,3,7,10-14H2,1-2H3,(H,27,28)/t15-,17-,18-,19-,20-/m0/s1
|
|
| 化学名 |
(2S,3aS,6aS)-1-[(2S)-2-[[(2S)-1-ethoxy-1-oxo-4-phenylbutan-2-yl]amino]propanoyl]-3,3a,4,5,6,6a-hexahydro-2H-cyclopenta[b]pyrrole-2-carboxylic acid
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 3.25 mg/mL (7.80 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 32.5 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 3.25 mg/mL (7.80 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 32.5 mg/mL 澄清 DMSO 储备液加入 900 μL 20% SBE-β-CD 生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 3.25 mg/mL (7.80 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 30% PEG400+0.5% Tween80+5% Propylene glycol: 30 mg/mL 配方 5 中的溶解度: 20 mg/mL (48.02 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶. 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.4009 mL | 12.0045 mL | 24.0090 mL | |
| 5 mM | 0.4802 mL | 2.4009 mL | 4.8018 mL | |
| 10 mM | 0.2401 mL | 1.2005 mL | 2.4009 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Ramipril 10 mg Capsule in Healthy Subjects Under Fasting Conditions
CTID: NCT00829452
Phase: Phase 1 Status: Completed
Date: 2024-08-19