| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg | |||
| Other Sizes |
| 靶点 |
human GnRH ( IC50 = 0.33 nM ); monkey GnRH ( IC50 = 0.32 nM )
|
|---|---|
| 体外研究 (In Vitro) |
Relugolix 对猴受体表现出与人类受体 (IC50=0.33 nM) 相当的强结合亲和力 (IC50=0.32 nM),而对大鼠受体 (IC50=9800 nM) 则降低了 30000 倍。在 40% 血清存在下,TAK-385 对人受体的体外拮抗活性 (IC90=18 nM) 比对猴受体 (IC90=1700 nM) 的拮抗活性高出 95 倍[1]。
|
| 体内研究 (In Vivo) |
Relugolix(口服;1-3 mg/kg;单剂量用于药代动力学研究)在 1 mg/kg 剂量下表现出良好的药代动力学特征和明显的对猴子循环 LH 水平的抑制作用。雄性食蟹猴的药代动力学曲线显示,Cmax、Tmax 和 AUCo 分别为 16.0 ng/mL、2.7 h 和 90.1 ng[1]。 Relugolix(口服;3、10 或 30 mg/kg;每天两次;4 周)显着降低男性睾丸重量,并在 3 mg/kg 时降低腹侧前列腺重量,在 10 mg/kg 时将其降低至去势水平hGNRHR 敲入小鼠[2]。 Relugolix(口服;30、100 或 200 mg/kg;每天两次;4 周)在 100 mg/kg 剂量的第一周内诱导所有小鼠持续绝情期,并在该剂量后显着降低卵巢和子宫的重量雌性 hGNRHR 敲入小鼠 4 周[2]。动物模型:雄性hGNRHR敲入小鼠[2] 剂量:3、10或30 mg/kg 给药方法:口服; 3、10或30毫克/公斤;每天两次; 4 周 结果:睾丸功能下降。动物模型:雌性 hGNRHR 敲入小鼠[2] 剂量:30、100 或 200 mg/kg 给药方法:口服;给药方式: 30、100或200毫克/公斤;每天两次; 4 周结果:将下丘脑-垂体-性腺轴抑制至性腺切除水平。垂体中 GnRH 受体 mRNA 水平下调。
|
| 动物实验 |
Male hGNRHR-knock-in mice
3, 10 or 30 mg/kg Oral administration; 3, 10 or 30 mg/kg; twice daily; 4 weeks |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
The Cmax and AUC of orally-administered relugolix increase proportionally following single doses - in contrast, with repeat dosing the AUC remains proportional to the dose while the Cmax increases greater than proportionally to the dose. Following the administration of 120mg once daily, the steady-state AUC and Cmax of relugolix were 407 (± 168) ng.hr/mL and 70 (± 65) ng/mL, respectively. The absolute oral bioavailability of relugolix is approximately 12% and the median Tmax following oral administration is 2.25 hours. Approximately 81% of an orally administered dose was recovered in the feces, of which 4.2% was unchanged parent drug, while 4.1% of the dose was recovered in the urine, of which 2.2% remained unchanged. The average renal clearance of relugolix is 8 L/h with a total clearance of 26.4 L/h. Metabolism / Metabolites Relugolix is metabolized mainly by the CYP3A subfamily of P450 enzymes, with a smaller contribution by CYP2C8. Biological Half-Life The average effective half-life of relugolix is 25 hours, while the average terminal elimination half-life is 60.8 hours. |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
Relugolix therapy has been associated with serum aminotransferase elevations above 3 times the upper limit of normal (ULN) in 1% to 3% of patients and in similar proportions of patients receiving comparator agents such as leuprolide or degarelix. The serum enzyme elevations are generally mild and self-limited, resolving even without dose adjustment. ALT values above 5 times the ULN occur in less than 1% of patients, and episodes of ALT elevations with symptoms or jaundice were not observed in preregistration clinical trials of relugolix alone or in combination with estradiol and norethindrone. Since its approval and more widescale use, there have been no published reports of clinically apparent liver injury attributed to relugolix. Likelihood score: E (unlikely cause of clinically apparent liver injury). Protein Binding Relugolix is 68-71% protein-bound in plasma, primarily to albumin and, to a lesser extent, α1-acid glycoprotein. |
| 参考文献 |
|
| 其他信息 |
Pharmacodynamics
Approximately 56% of patients achieved castrate-level testosterone concentrations (<50 ng/dL) by day 4 of therapy and 97% of patients maintain these levels through 48 weeks of therapy. Relugolix requires once-daily oral administration to maintain the desired testosterone concentrations. Androgen deprivation therapies may prolong the QTc interval and should therefore be used with caution in patients having a high baseline risk of QTc prolongation, such as those with electrolyte abnormalities, congestive heart failure, or using other medications known to prolong the QTc interval. Based on its mechanism of action and data from animal studies, relugolix may result in fetal harm if administered to pregnant females - male patients with female partners should be advised to use effective contraception throughout therapy and for 2 weeks following cessation of therapy to prevent inadvertent fetal exposure. |
| 分子式 |
C29H27F2N7O5S
|
|---|---|
| 分子量 |
623.630391359329
|
| 精确质量 |
623.18
|
| 元素分析 |
C, 55.85; H, 4.36; F, 6.09; N, 15.72; O, 12.83; S, 5.14
|
| CAS号 |
737789-87-6
|
| 相关CAS号 |
Relugolix-d6
|
| PubChem CID |
10348973
|
| 外观&性状 |
White to off-white solid powder
|
| LogP |
3.966
|
| tPSA |
169.36
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
11
|
| 可旋转键数目(RBC) |
9
|
| 重原子数目 |
44
|
| 分子复杂度/Complexity |
1010
|
| 定义原子立体中心数目 |
0
|
| SMILES |
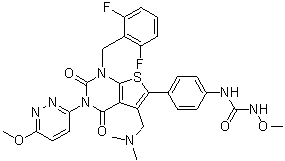
O=C(NOC)NC1=CC=C(C(S2)=C(CN(C)C)C(C(N3C4=NN=C(OC)C=C4)=O)=C2N(CC5=C(F)C=CC=C5F)C3=O)C=C1
|
| InChi Key |
AOMXMOCNKJTRQP-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C29H27F2N7O5S/c1-36(2)14-19-24-26(39)38(22-12-13-23(42-3)34-33-22)29(41)37(15-18-20(30)6-5-7-21(18)31)27(24)44-25(19)16-8-10-17(11-9-16)32-28(40)35-43-4/h5-13H,14-15H2,1-4H3,(H2,32,35,40)
|
| 化学名 |
1-[4-[1-[(2,6-difluorophenyl)methyl]-5-[(dimethylamino)methyl]-3-(6-methoxypyridazin-3-yl)-2,4-dioxothieno[2,3-d]pyrimidin-6-yl]phenyl]-3-methoxyurea
|
| 别名 |
TAK 385; TAK385; TAK-385; trade names: Orgovyx; Relumina
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: 61~100 mg/mL (97.8~160.4 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 0.83 mg/mL (1.33 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 8.3 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 0.83 mg/mL (1.33 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 8.3 mg/mL 澄清 DMSO 储备液加入 900 μL 20% SBE-β-CD 生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 0.83 mg/mL (1.33 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.6035 mL | 8.0176 mL | 16.0351 mL | |
| 5 mM | 0.3207 mL | 1.6035 mL | 3.2070 mL | |
| 10 mM | 0.1604 mL | 0.8018 mL | 1.6035 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
LIBERTY 1: An International Phase 3 Randomized, Double-Blind, Placebo-Controlled Efficacy and Safety Study to Evaluate Relugolix Co Administered with and without Low-Dose Estradiol and Norethindrone Acetate in Women with Heavy Menstrual Bleeding Associated with Uterine Fibroids
CTID: null
Phase: Phase 3 Status: Completed
Date: 2017-06-12