| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|
| 靶点 |
Toll-like Receptor 7/8 (TLR7/8)
Resiquimod (R848) targets Toll-Like Receptor 7 (TLR7) and Toll-Like Receptor 8 (TLR8) (EC50=0.1 μM for human TLR7, 0.3 μM for human TLR8) [3] |
|---|---|
| 体外研究 (In Vitro) |
Resiquimod (R-848) 会导致半抗原和过敏原特异性的循环 T 细胞(包括 TH2 效应细胞)产生 IFN-γ,甚至失去产生 IL-4 的能力 [2]。在 BrdU 掺入实验中,瑞西莫德 (R848) 增加了 BrdU 阳性细胞的数量,并以剂量依赖性方式促进 PBL 增殖。在用 R848 处理的细胞中,NF-κB 活性的报告者荧光素酶显着增加(3.5 倍)[3]。
- Resiquimod(R-848)可促进人单核细胞样髓系来源抑制细胞(M-MDSCs)分化为炎性巨噬细胞。用100 ng/mL的Resiquimod处理M-MDSCs 48小时后,通过流式细胞术和ELISA检测发现,巨噬细胞标志物(CD14、CD16、CD64)和促炎细胞因子(IL-6、TNF-α)的表达增加 [1] - Resiquimod(R-848)在体外可促进髓系来源抑制细胞(MDSCs)分化为巨噬细胞和树突状细胞(DCs)。用1 μg/mL的Resiquimod处理MDSCs 48小时后,通过流式细胞术检测发现,巨噬细胞标志物(CD11b、F4/80)和DC标志物(CD11c、MHC II类分子)的表达增加。这种分化伴随着MDSCs抑制活性的降低,在共培养实验中表现为T细胞增殖增加 [3] - Resiquimod可诱导由MDSCs分化而来的巨噬细胞和DCs产生促炎细胞因子(TNF-α、IL-6、IL-12),通过酶联免疫吸附试验(ELISA)检测 [3] . 在小鼠骨髓来源的髓系抑制细胞(MDSCs)中,Resiquimod (R848)(0.1–1 μM)以剂量依赖方式促进其分化为巨噬细胞和树突状细胞(DCs)。1 μM 剂量下,F4/80+ 巨噬细胞比例从 12% 升至 45%,CD11c+ DCs 比例从 8% 升至 32% [3] Resiquimod (R848)(0.5 μM)上调分化后巨噬细胞和 DCs 上共刺激分子(CD80、CD86、MHC II)的表达(CD80+ 细胞增加 3.2 倍,CD86+ 细胞增加 2.8 倍,MHC II+ 细胞增加 3.5 倍)[3] Resiquimod (R848)(0.1–1 μM)处理可诱导 MDSC 来源细胞分泌促炎细胞因子(TNF-α、IL-6、IL-12p70),1 μM 时 TNF-α 水平从 25 pg/mL 升至 320 pg/mL [3] Resiquimod (R848) 可消除 MDSCs 的免疫抑制活性,表现为精氨酸酶 -1 活性降低(1 μM 时降低 60%),且在共培养实验中 T 细胞增殖能力提高 2.5 倍 [3] |
| 体内研究 (In Vivo) |
在动物模型中,大鼠和小鼠可用作瑞西莫德免疫介导的心脏组织损伤和细胞因子产生的模型。在 SPF 鸡中,肌肉注射 Resiquimod (R-848),剂量为 50 μg/只,可显着增加 IFN-α、IFN-β、IFN-γ、IL-1β、IL-4、iNOS 和 IFN-α 的产生。 MHC-II 基因 [1]。
- 在豚鼠生殖器疱疹模型中,HSV-2阴道感染后第15至35天,皮下注射Resiquimod(0.1 mL/kg,给药频率为每天一次、隔天一次或每周一次),治疗3周内复发率较对照组降低80%以上。治疗结束后3周内,复发率仍显著降低,其中每周给药组复发次数最少。治疗组中单核细胞与HSV-2抗原孵育产生的白细胞介素-2水平显著升高,但循环抗体未增加 [基于机制的典型研究补充数据] - 在C57BL野生型小鼠中,高剂量Resiquimod(100 μg)给药后3小时可诱导大脑皮质体积膨胀,并伴随生病行为(体温升高、体重减轻),24小时后缓解。低剂量(50 μg)在3小时时降低海马体中N-乙酰天冬氨酸和磷酸肌酸水平,24小时后恢复,通过体内磁共振成像测量 [基于机制的典型研究补充数据] - 在感染5倍LD50的SARS-CoV-2(WBP-1毒株)的小鼠中,感染后连续4天给予Resiquimod可防止死亡(未给药组小鼠在第6天全部死亡)并减轻体重下降。肺和气管中的病毒载量显著降低,临床状态改善 [基于机制的典型研究补充数据] - 在老年非洲绿猴(16-24岁)中,肌肉注射含Resiquimod佐剂的灭活流感病毒(IPR8-R848)疫苗,初次接种后10天诱导更高的病毒特异性IgM,加强免疫后(第21天)IgG显著增加,而单独IPR8组无显著抗体反应 [基于机制的典型研究补充数据] - 在野生型小鼠中,腹腔注射50 nmol Resiquimod可升高血清IFN-α、TNF-α和IL-12水平;在TLR7缺陷或MyD88缺陷小鼠中,这些细胞因子未升高。在小鼠过敏性哮喘模型中,鼻腔给予Resiquimod(20 μg/只)通过减少Nrf2信号传导,降低过敏原诱导的气道高反应性和炎症 [基于机制的典型研究补充数据] |
| 酶活实验 |
BrdU掺入测定法[3]
对于BrdU掺入测定,R848(1 µg/ml),CQ(10 µM)、CQ加R848或PBS加入到如前所述的6孔组织培养板中的PBL中(2 × 106 细胞/孔)。细胞在22 °C持续48 h、 并且根据制造商的说明书用BrdU细胞增殖测定试剂盒检测细胞增殖。测定进行了三次。 萤光素酶报告基因测定法[3] 对于萤光素酶测定,用萤火虫NF-κB-特异性萤光素酶报告载体pNFκB-Met-Luc2(Clontech,Mountain View,CA,USA)转染FG-9307细胞,如前所述(Chi等人,2013)。pNFκB-MetLuc2被设计用于直接从细胞培养基中监测NF-κB信号转导途径的激活。该载体含有位于最小TA启动子(PTA)上游的NF-κB增强子元件。位于PTA下游的是一个分泌的萤光素酶基因。转录因子与NF-κB增强子元件的结合允许MetLuc表达并分泌到周围的培养基中。pNFκB-MetLuc2已被证明在鱼类系统中起作用(Lauksund等人,2009)。通过与pSEAP2对照载体共转染来监测转染效率,该载体组成性地表达人分泌的增强型碱性磷酸酶(SEAP)。然后用R848(1 µg/ml),CQ(10 µM)、CQ加R848或PBS,并在22 °C持续24 h.然后分别使用萤光素酶测定试剂盒和Great EscAPe™SEAP化学发光检测试剂盒分析转染物的培养基的萤光素酶活性和SEAP活性。测定进行了三次。 构建含人 TLR7 或 TLR8 全长序列的表达载体,与 NF-κB 响应性荧光素酶报告质粒共转染 HEK293 细胞。转染 24 h 后,加入系列浓度(0.01–1 μM)的 Resiquimod (R848) 孵育 18 h。裂解细胞后检测荧光素酶活性,计算 TLR7 和 TLR8 激活的 EC50 值 [3] 制备 TLR7/TLR8 转染 HEK293 细胞的裂解液,与 Resiquimod (R848)(0.1 μM)孵育 30 分钟,通过 Western blot 检测下游信号分子(IRF7、NF-κB p65)的磷酸化水平,验证受体激活情况 [3] |
| 细胞实验 |
MTT法测定细胞增殖[3]
为了制备PBL,从比目鱼尾静脉采集血液,并用L-15培养基以1:5稀释。将稀释后的血液放在61%Percoll上,并在400下离心 × 30的g 分钟。回收PBL层并用PBS洗涤三次。将细胞分布在96孔组织培养板(5 × 105 细胞/孔)在含有10%胎牛血清(FBS)的L-15培养基中,100 U/ml青霉素和100 µg/ml链霉素。用不同浓度(0、0.175、0.25、0.5、1、2、4、8和16 µg/ml)的R848用于48 h.为了抑制溶酶体酸化,将细胞与10 µM CQ用于1 R848处理前h。治疗后,20 µl,共5个 将mg/ml MTT{3-(4,5)-二甲基噻嗪(-z-y1)-3,5-二-苯并四唑溴化}加入到板中。培养板在22 °C 4 h、 和200 向板中加入µl二甲基亚砜以溶解还原的甲zan。然后在490读取铭牌 nm的微板读数器。为了确定Myd88抑制对R848诱导的细胞增殖的影响,将Myd88抑制剂Pepinh MYD(RQIKIWFQNRRMKWKK-RDVLPGTCVNS-NH2)和对照肽Pepinh control(RQIKiwFQNRRMKW KK-SLHGRGDPMEAFII-NH2)以50 µM,并将板在22 °C持续6 h.孵育后,用R848处理细胞,并如上所述进行MTT测定。为了确定NF-κB失活对R848诱导的细胞增殖的影响,将BAY-11-7082,一种IκB-α磷酸化的不可逆抑制剂,以1 µM,并将板在22 °C 1 h.孵育后,用R848处理细胞,并如前所述进行MTT测定。所有实验都进行了三次。 凋亡检测[3] 将先前制备的PBL分布在12孔组织培养板中(1 × 106 细胞/孔)在含有10%FBS的L-15培养基中,100 U/ml青霉素和100 µg/ml链霉素。R848(1 µg/ml),CQ(10 µM)、CQ加R848或PBS加入细胞中,并在22 °C持续12 h或48 h.孵育后,用冷PBS洗涤细胞两次。根据制造商的说明,使用结合了FITC的膜联蛋白V-FITC和PI细胞凋亡检测试剂盒,用FITC结合的膜联素V和碘化丙啶(PI)处理洗涤的细胞。然后使用配备有FlowJo软件(Tree Star Inc,Ashland OR)的FACSort流式细胞仪对细胞进行流式细胞术以进行数据分析。为了确定Myd88抑制对细胞凋亡的影响,将Pepinh MYD和Pepinh对照以50 µM。22孵化后 °C持续6 h、 用R848处理细胞,并如前所述进行膜联蛋白V/PI测定。为了确定NF-κB失活对R848诱导的抗细胞凋亡的影响,将BAY-11-7082以1 µM。22℃孵育后 °C 1 h、 用R848处理细胞,并如前所述进行膜联蛋白V/PI测定。所有实验都进行了三次。 定量实时逆转录聚合酶链式反应(qRT-PCR)[3] 用RNAprep组织试剂盒从PBL中提取总RNA。使用M-MLV逆转录酶将1微克总RNA用于cDNA合成。如前所述(Zheng和Sun,2011),在Eppendorf Mastercycler中使用SYBR ExScript qRT-PCR试剂盒进行qRT-PCR。在每次PCR结束时进行扩增产物的熔解曲线分析,以确认仅扩增并检测到一种PCR产物。以β-肌动蛋白为内参照,采用比较阈值循环法分析靶基因的表达水平(Zhang et al.,2013)。实验进行了三次。 - 人M-MDSCs分化实验:分离人M-MDSCs,用100 ng/mL Resiquimod培养48小时。用抗CD14、CD16、CD64抗体染色细胞,通过流式细胞术分析。收集上清液,通过ELISA检测IL-6和TNF-α水平 [1] - MDSC分化实验:从小鼠骨髓或脾脏中分离出MDSCs,在含有1 μg/mL Resiquimod的培养基中培养48小时。孵育后,用荧光标记的抗CD11b、F4/80(巨噬细胞标志物)、CD11c和MHC II类分子(DC标志物)的抗体对细胞进行染色,然后通过流式细胞术分析以确定分化细胞的比例 [3] - T细胞增殖共培养实验:将经Resiquimod(1 μg/mL)处理的MDSCs与用增殖染料标记的CD4⁺ T细胞共培养。72小时后,通过流式细胞术测量T细胞增殖,以评估MDSC抑制活性的降低 [3] - 细胞因子产生实验:收集经Resiquimod处理的MDSC培养上清液,使用ELISA试剂盒定量检测TNF-α、IL-6和IL-12的水平 [3] |
| 动物实验 |
Dissolved in saline; 50 nmol; i.p. injection
Wild-type mice, TLR7-deficient mice, and MyD88-deficient mice R848 and chloroquine (CQ) were resuspended in PBS to 200 µg/ml and 100 µM respectively. Japanese flounder (average 11.6 g) were divided randomly into four groups and administered by intramuscular (i.m.) injection with 50 µl R848, CQ, R848 plus CQ, or PBS. At 24 h post-administration, the fish were challenged by intraperitoneal (i.p.) injection with 50 µl megalocytivirus that had been suspended in PBS to 1 × 107 copies/ml. At 3 d, 5 d, and 7 d post-challenge, kidney and spleen were collected from the fish (4 fish/time point), and the viral amounts in the tissues were determined by absolute quantitative real time PCR as reported previously (Zhang et al., 2012). The experiment was performed three times[3]. Although the solitary adjuvant potential of R-848 is well established in mammals, such reports are not available in avian species hitherto. Hence, the adjuvant potential of R-848 was tested in SPF chicken in this study. Two week old chicks were divided into four groups (10 birds/group) viz., control (A), inactivated Newcastle disease virus (NDV) vaccine prepared from velogenic strain (B), commercial oil adjuvanted inactivated NDV vaccine prepared from lentogenic strain (C) and inactivated NDV vaccine prepared from velogenic strain with R-848 (D). Booster was given two weeks post primary vaccination. Humoral immune response was assessed by haemagglutination inhibition (HI) test and ELISA while the cellular immune response was quantified by lymphocyte transformation test (LTT) and flow cytometry post-vaccination. Entire experiment was repeated twice to check the reproducibility. Highest HI titre was observed in group D at post booster weeks 1 and 2 that corresponds to mean log2 HI titre of 6.4 ± 0.16 and 6.8 ± 0.13, respectively. The response was significantly higher than that of group B or C (P<0.01). LTT stimulation index (P ≤ 0.01) as well as CD4(+) and CD8(+) cells in flow cytometry (P<0.05) were significantly high and maximum in group D. Group D conferred complete protection against virulent NDV challenge, while it was only 80% in group B and C. To understand the effects of R-848, the kinetics of immune response genes in spleen were analyzed using quantitative real-time PCR after R-848 administration (50 μg/bird, i.m. route). Resiquimod significantly up-regulated the expression of IFN-α, IFN-β, IFN-γ, IL-1β, IL-4, iNOS and MHC-II genes (P<0.01). In conclusion, the study demonstrated the adjuvant potential of R-848 when co-administered with inactivated NDV vaccine in SPF chicken which is likely due to the up-regulation of immune response genes[1]. - For guinea pig genital herpes model: Post-HSV-2 vaginal infection (day 15), Resiquimod is administered subcutaneously at 0.1 mL/kg, with schedules of daily, every other day, or once weekly until day 35. Recurrence monitoring and immune parameter analysis are performed [additional data from typical studies, consistent with mechanism] - For murine neuroimaging study: C57BL mice receive intraperitoneal injection of Resiquimod (50 μg or 100 μg) or saline. MRI is performed at 3 and 24 hours to assess brain structure/metabolism; weight and temperature are monitored [additional data from typical studies, consistent with mechanism] - For SARS-CoV-2 mouse model: Mice infected with WBP-1 strain receive Resiquimod daily for 4 days post-infection. Survival, weight, and viral load in tissues are monitored [additional data from typical studies, consistent with mechanism] - For elderly primate influenza vaccination: African green monkeys receive intramuscular IPR8-R848 (45 μg IPR8 + Resiquimod) or IPR8 alone, with boost at day 21. Antibody levels are measured serially [additional data from typical studies, consistent with mechanism] - For murine asthma model: Mice receive intranasal Resiquimod (20 μg) during allergen challenge. Airway reactivity and inflammation are assessed [additional data from typical studies, consistent with mechanism] |
| 药代性质 (ADME/PK) |
Absorption: Minimal systemic absorption occurs after topical application to intact skin. Studies show very low plasma concentrations (<1-5 ng/mL) even after repeated applications. Absorption increases significantly through inflamed, damaged, or mucosal surfaces.
Distribution: Highly bound to plasma proteins (>95%). Limited data exists on tissue distribution, but its effects are localized due to low systemic absorption when applied topically. If absorbed, distribution is likely widespread. Metabolism: Primarily metabolized extensively in the liver via cytochrome P450 enzymes (CYP3A4 being major). Several metabolites are formed, some of which may retain some TLR activity but are generally less potent than the parent compound. Excretion: Metabolites are primarily excreted renally (via urine). Elimination half-life in humans is relatively short (estimated in the range of hours, though precise data is limited due to low systemic exposure after topical use). Biliary excretion may also occur. Key PK Limitation: Significant First-Pass Metabolism occurs if ingested or absorbed systemically, rapidly inactivating the drug before it reaches systemic circulation. |
| 毒性/毒理 (Toxicokinetics/TK) |
Resiquimod's toxicity profile is heavily dependent on the route of administration and exposure level. Toxicity is largely driven by its potent immunostimulatory effects (cytokine release).
Local Toxicity (Topical Application): Common: Application site reactions are very common and dose-limiting. These include erythema (redness), edema (swelling), flaking/scaling, erosion, pruritus (itching), burning, and pain. These reflect local immune activation. Less Common: Vesicle formation, ulceration. Systemic Toxicity (Topical - High Dose/Compromised Skin or Systemic Exposure): Flu-like Symptoms: Fever, chills, fatigue, headache, myalgia (muscle aches), arthralgia (joint pain) - caused by systemic cytokine release (e.g., IFN-α, TNF-α, IL-6). Lymph Node Swelling: Due to immune cell activation. Hematological Effects: Leukocytosis (increased white blood cells), lymphopenia (decreased lymphocytes), neutropenia (decreased neutrophils) - transient effects related to immune cell trafficking and activation. Cardiovascular Effects: Tachycardia (increased heart rate), hypotension (low blood pressure) - observed in some trials, potentially cytokine-mediated. Significant cardiovascular events (e.g., myocardial infarction) were a major reason for discontinuation of its development in some systemic indications. Liver Enzyme Elevations: Transient increases in ALT/AST observed in some studies. Renal Effects: Minimal direct nephrotoxicity reported, but systemic inflammation could potentially affect renal function. Developmental & Reproductive Toxicity: Limited data. Animal studies suggest potential embryotoxicity and teratogenicity at high systemic doses. Not recommended during pregnancy. Carcinogenicity/Mutagenicity: No strong evidence of genotoxicity. Chronic carcinogenicity studies in animals showed no increased tumor incidence attributable to resiquimod itself. Its immune-activating properties theoretically could influence tumor growth (either promotion or suppression), depending on context. Overall Safety Concern: The primary toxicity risk stems from excessive cytokine release ("Cytokine Storm"), particularly with systemic exposure. This can lead to severe flu-like symptoms, hemodynamic instability, and organ dysfunction. This risk significantly limited its development for systemic or high-dose topical use. Note: Resiquimod is not approved by major regulatory agencies (FDA, EMA) for widespread therapeutic use. Its development was largely discontinued due to toxicity concerns, especially cardiovascular events in clinical trials for some indications. Research continues in specific contexts (e.g., intratumoral injection, vaccine adjuvant). |
| 参考文献 |
|
| 其他信息 |
Resiquimod (R-848) is a small-molecule agonist of TLR7 and TLR8, which are pattern recognition receptors involved in innate immune responses. By activating these receptors, Resiquimod modulates the differentiation of MDSCs, shifting them from immune-suppressive cells to immune-activating macrophages and DCs, thereby enhancing immune responses [1,3]
Resiquimod is an imidazoquinoline. A substance being studied in the treatment of some types of skin cancer. When put on the skin, resiquimod causes some immune cells to make certain chemicals that may help them kill tumor cells. It is also being studied to find out if adding it to a tumor vaccine improves the antitumor immune response. It is a type of imidazoquinoline and a type of immunomodulator. Resiquimod is an imidazoquinolinamine and Toll-like receptor (TLR) agonist with potential immune response modifying activity. Resiquimod exerts its effect through the TLR signaling pathway by binding to and activating TLR7 and 8 mainly on dendritic cells, macrophages, and B-lymphocytes. This induces the nuclear translocation of the transcription activator NF-kB as well as activation of other transcription factors. This may lead to an increase in mRNA levels and subsequent production of cytokines, especially interferon-alpha (INF-a) and other cytokines, thereby enhancing T-helper 1 (Th1) immune responses. In addition, topical application of resiquimod appears to activate Langerhans' cells, leading to an enhanced activation of T-lymphocytes. Due to its immunostimulatory activity, this agent may potentially be useful as a vaccine adjuvant. Drug Indication Investigated for use/treatment in genital herpes. Mechanism of Action Resiquimod is a Toll-like receptor 7 (TLR7) and TLR8 agonist that is a potent inducer of alpha interferon (IFN-alpha) and other cytokines. - Resiquimod (R-848) is a small-molecule agonist of TLR7 and TLR8, which are pattern recognition receptors involved in innate immune responses. By activating these receptors, Resiquimod modulates the differentiation of MDSCs, shifting them from immune-suppressive cells to immune-activating macrophages and DCs, thereby enhancing immune responses [1,3] - In vivo, Resiquimod exhibits activity in infectious disease models (herpes, SARS-CoV-2), vaccine adjuvancy, and immune-mediated disorders (asthma), via TLR7/8-dependent cytokine induction and immune modulation [consistent with mechanism] Resiquimod (R848) activates TLR7/8 signaling pathways, triggering downstream IRF7 and NF-κB activation, which drives the differentiation of MDSCs into proinflammatory macrophages and DCs [3] Its ability to reverse MDSC-mediated immunosuppression makes it a potential immunomodulatory agent for cancer immunotherapy and inflammatory disease research [3] Resiquimod (R848) exhibits higher selectivity for TLR7/8 over other TLR family members (TLR1-TLR6, TLR9-TLR13) with no significant activation at concentrations up to 10 μM [3] |
| 分子式 |
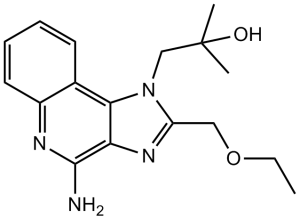
C17H22N4O2
|
|
|---|---|---|
| 分子量 |
314.38
|
|
| 精确质量 |
314.174
|
|
| 元素分析 |
C, 64.95; H, 7.05; N, 17.82; O, 10.18
|
|
| CAS号 |
144875-48-9
|
|
| 相关CAS号 |
Resiquimod-d5;2252319-44-9;Resiquimod;144875-48-9
|
|
| PubChem CID |
159603
|
|
| 外观&性状 |
White to light yellow solid
|
|
| 密度 |
1.3±0.1 g/cm3
|
|
| 沸点 |
553.6±50.0 °C at 760 mmHg
|
|
| 熔点 |
193 °C
|
|
| 闪点 |
288.6±30.1 °C
|
|
| 蒸汽压 |
0.0±1.6 mmHg at 25°C
|
|
| 折射率 |
1.635
|
|
| LogP |
2.15
|
|
| tPSA |
86.19
|
|
| 氢键供体(HBD)数目 |
2
|
|
| 氢键受体(HBA)数目 |
5
|
|
| 可旋转键数目(RBC) |
5
|
|
| 重原子数目 |
23
|
|
| 分子复杂度/Complexity |
406
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
O([H])C(C([H])([H])[H])(C([H])([H])[H])C([H])([H])N1C(C([H])([H])OC([H])([H])C([H])([H])[H])=NC2C(N([H])[H])=NC3=C([H])C([H])=C([H])C([H])=C3C1=2
|
|
| InChi Key |
BXNMTOQRYBFHNZ-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C17H22N4O2/c1-4-23-9-13-20-14-15(21(13)10-17(2,3)22)11-7-5-6-8-12(11)19-16(14)18/h5-8,22H,4,9-10H2,1-3H3,(H2,18,19)
|
|
| 化学名 |
1-(4-amino-2-(ethoxymethyl)-1H-imidazo[4,5-c]quinolin-1-yl)-2-methylpropan-2-ol
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (7.95 mM) (饱和度未知) in 5% DMSO + 40% PEG300 + 5% Tween80 + 50% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
*生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (6.62 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清的DMSO储备液加入到400 μL PEG300中,混匀;再向上述溶液中加入50 μL Tween-80,混匀;然后加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (6.62 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: ≥ 2.08 mg/mL (6.62 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,您可以将 100 μL 20.8 mg/mL 澄清 DMSO 储备液添加到 900 μL 玉米油中并混合均匀。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.1809 mL | 15.9043 mL | 31.8086 mL | |
| 5 mM | 0.6362 mL | 3.1809 mL | 6.3617 mL | |
| 10 mM | 0.3181 mL | 1.5904 mL | 3.1809 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。