| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 2mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| Other Sizes |
|
| 靶点 |
THR-β (EC50 = 0.21 μM)
|
||
|---|---|---|---|
| 体外研究 (In Vitro) |
与 THR-α (EC50=3.74 μM) 相比,Resmetirom (MGL-3196) 对 THR-β (EC50=0.21 μM) 的选择性提高了 28 倍。 Resmetirom (MGL-3196) 用于抑制 hERG 通道,IC20 约为 30 μM。对CYP2C9的抑制作用相对温和(约22μM),而CYP3A4/5和CYP2C19的IC50>50μM[1]。
|
||
| 体内研究 (In Vivo) |
在大鼠中,瑞美罗 (MGL-3196) 显示出合理的口服生物利用度和良好的暴露量。分布量少,出清少。口服 Resmetirom (MGL-3196) 溶液后,DIO 小鼠中发现暴露量与剂量成比例增加 [1]。由于肝脏 TG,给予 Resmetirom (MGL-3196) 的大鼠胆固醇和肝脏大小有所减少。使用 Resmetirom (MGL-3196) 治疗的动物心脏或肾脏大小或骨矿物质密度 (BMD) 没有变化 [1]。
雷美替隆治疗不影响体重,但导致肝脏重量、肝脂肪变性、血浆丙氨酸氨基转移酶活性、肝脏和血浆胆固醇以及血糖显著降低。这些代谢效应转化为NAFLD活动评分的显著改善。此外,α-平滑肌肌动蛋白含量较低和参与纤维化的基因下调表明肝纤维化减少。[2] |
||
| 酶活实验 |
THR/RXR/GRIP1 Assay1]
将THR-β(H6-THR-β)的配体结合结构域(氨基酸148-410)和THR-α(H6-THR-α)的配体连接结构域(氨基202-461)克隆到含有N端六His序列的大肠杆菌表达载体pET28a中。在大肠杆菌BL21(DE3)细胞中产生了重组六His标记蛋白。使用摇瓶在25°C下在0.2 mM IPTG中诱导24小时,在Terrific Broth(内部制备的杆菌胰蛋白胨(3.3%,w/v)、Difico酵母提取物(2.0%,w/v”)和NaCl(0.5%,w/v“)培养基中培养细胞,收获细胞,并用五倍体积的缓冲液a(0.05 M Tris、0.3 M NaCl、1%w/v甜菜碱、0.01 M咪唑、0.02 Mβ-巯基乙醇,pH 8.0)裂解。将溶菌酶(1.0 mg/mL)和完全蛋白酶抑制剂混合物加入浆液中,在4°C下对溶液进行5次超声波处理1分钟。将悬浮液在Ti45 Beckmann转子中以127 300 RCF离心2小时,将上清液装载到NI_NTA琼脂糖(Quigen 30210)柱上。用缓冲液a洗涤后,用含有0.25M咪唑的缓冲液a洗脱H6-TRβ或H6-TRα。[1] 人维甲酸X受体(氨基酸225-462)(RxRα)的配体结合结构域用N端His6和EE(EFMPME)标签工程化,这是His6和EE序列之间的凝血酶切割位点,并克隆到pACYC载体中。在大肠杆菌细胞中产生了His6-EE标记的蛋白质。使用摇瓶在18°C下在0.1 mM IPTG中培养18小时,收获细胞,并用五倍体积的缓冲液B(0.025 M Tris、0.3 M NaCl、0.02 M咪唑、0.01 Mβ-巯基乙醇,pH 8.0)悬浮。加入溶菌酶(0.2 mg/mL)和完全蛋白酶抑制剂混合物,并在4°C下搅拌30分钟。将悬浮液在4°C下超声处理30秒,共五次。将悬浮液在12000 RCF下离心20分钟。上清液用0.45μm孔径的膜过滤,加入0.5%的NP-40。His6标记的蛋白与NiNTA金属亲和树脂结合并从中洗脱。将蛋白质浓缩并透析。 通过凝血酶消化从EE RxRα中去除His6标签,每毫克蛋白质使用10单位凝血酶(Pharmacia,Piscataway,NJ),在25°C下孵育2小时。使用苯甲脒琼脂糖6B分批去除凝血酶。将蛋白质浓缩并透析。该蛋白用于辅活化剂肽募集试验。[1] THR-β/RXR/GRIP1共激活肽募集试验[1] 将30μL H6-THR-β(50 nM)在50 mM Hepes、pH 7.0、1 mM DTT、0.05%NP40和0.2 mg/mL BSA(结合缓冲液)中与等体积的EE RxRα(50 nM)在结合缓冲液中混合。然后加入6μL的T3(0-14.8μM)或测试化合物(0-1.2 mM)的DMSO溶液,并在37°C下孵育30分钟。然后加入30μL的生物素-GRIP1肽(生物素-Aca-HGTSLKEKILHRLLQDSPSVDL-CONH2)(100 nM)的30μL结合缓冲液加5%的DMSO溶液并在37℃下孵育60分钟。加入30μL的溶液,其中含有12 nM铕偶联的抗hexa-His抗体和160 nM APC偶联的链霉抗生物素,溶于50 mM Tris、pH 7.4、100 mM NaCl和0.2 mg/mL BSA中,以及将溶液在4°C下孵育过夜。将等分试样(35μL/样品)转移到384孔黑色微量滴定板上。HTRF信号在Victor 5阅读器上读取。[1] THR-α/RXR/GRIP1共激活肽募集试验[1] 除了使用125 nM H6-THR-α、125 nM EE RXRα和250 nM生物素-GRIP1外,该测定方案与上述THR-β/RXR/GRIP1共激活肽募集测定基本相同。 |
||
| 动物实验 |
|
||
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
Mild, transient serum aminotransferase elevations develop in a high proportion of patients receiving resmetirom, generally within the first 4 weeks of therapy. These elevations are typically mild, self-limited, and not associated with symptoms or jaundice. Furthermore, these early changes were usually followed by a decrease in serum enzymes which were often within normal range 3 to 6 months later. These improvements in liver related enzymes correlated to some extent with the decrease in hepatic fat and histologic evidence of steatohepatitis. After 52 weeks of treatment, liver biopsies demonstrated resolution of NASH in 26% to 30% of patients. Whether these changes are sustained or increase with further therapy is not known. Therapy does not result in weight loss, and the improvements in hepatic histology and fibrosis may be lost once therapy is discontinued. Analysis of liver tests from more than 1300 adults with NASH treated with resmetirom in doses of 80 or 100 mg daily for up to one year identified 2 patients with liver injury that was considered at least possibly due to resmetirom. The latency to initial onset was 2 and 3 months [ALT 236 U/L and 578 U/L, Alk P unknown and 64 U/L, bilirubin 0.6 and 1.1 mg/dL]. Both patients recovered completely within 1 to 2 months of stopping treatment. One patient was restarted on treatment and redeveloped liver injury within 28 days (ALT 3226 U/L, Alk P 140 U/L, bilirubin 10.9 mg/dL) that was more severe than the initial episode, but that resolved spontaneously within 2 months of stopping. In both cases, other diagnoses remained possible. Likelihood score: D (possible rare cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation No information is available on the clinical use of resmetirom during breastfeeding. Because resmetirom is more than 99% bound to plasma proteins, the amount in milk is likely to be low. If the mother requires resmetirom, it is not a reason to discontinue breastfeeding. Until more data are available, resmetirom should be used with careful infant monitoring during breastfeeding, especially while nursing a newborn or preterm infant. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. |
||
| 参考文献 |
[1]. Discovery of 2-[3,5-dichloro-4-(5-isopropyl-6-oxo-1,6-dihydropyridazin-3-yloxy)phenyl]-3,5-dioxo-2,3,4,5-tetrahydro[1,2,4]triazine-6-carbonitrile (MGL-3196), a Highly Selective Thyroid Hormone Receptor β agonist in clinical trials for the treatment of dyslipidemia. J Med Chem. 2014 May 22;57(10):3912-23.
[2]. Activation of thyroid hormone receptor-β improved disease activity and metabolism independent of body weight in a mouse model of non-alcoholic steatohepatitis and fibrosis. Br J Pharmacol . 2021 Jun;178(12):2412-2423. [3]. A new mechanism of thyroid hormone receptor β agonists ameliorating nonalcoholic steatohepatitis by inhibiting intestinal lipid absorption via remodeling bile acid profiles. Acta Pharmacologica Sinica (2024). |
||
| 其他信息 |
MGL-3196 has been used in trials studying the treatment of Non-alcoholic steatohepatitis and Heterozygous Familial Hypercholesterolemia.
Resmetirom is a thyroid hormone receptor beta (THR-β) agonist used in conjunction with diet and exercise in the therapy of nonalcoholic steatohepatitis (NASH) with moderate-to-severe fibrosis. Resmetirom therapy is associated with mild and transient serum aminotransferase elevations during the first month of therapy and with rare instances of acute liver injury which can be severe, but which reverses on drug discontinuation. Drug Indication Treatment of non-alcoholic steatohepatitis (NASH) |
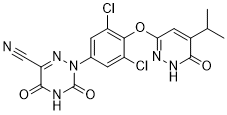
| 分子式 |
C17H12CL2N6O4
|
|
|---|---|---|
| 分子量 |
435.22
|
|
| 精确质量 |
434.03
|
|
| 元素分析 |
C, 46.92; H, 2.78; Cl, 16.29; N, 19.31; O, 14.70
|
|
| CAS号 |
920509-32-6
|
|
| 相关CAS号 |
|
|
| PubChem CID |
15981237
|
|
| 外观&性状 |
Typically exists as Yellow to orange solids at room temperature
|
|
| 密度 |
1.65±0.1 g/cm3 (20 °C, 760 mmHg)
|
|
| LogP |
2.098
|
|
| tPSA |
146.52
|
|
| 氢键供体(HBD)数目 |
2
|
|
| 氢键受体(HBA)数目 |
7
|
|
| 可旋转键数目(RBC) |
4
|
|
| 重原子数目 |
29
|
|
| 分子复杂度/Complexity |
878
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
N#CC1C(=O)NC(=O)N(C2C=C(Cl)C(OC3C=C(C(C)C)C(=O)NN=3)=C(Cl)C=2)N=1
|
|
| InChi Key |
FDBYIYFVSAHJLY-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C17H12Cl2N6O4/c1-7(2)9-5-13(22-23-15(9)26)29-14-10(18)3-8(4-11(14)19)25-17(28)21-16(27)12(6-20)24-25/h3-5,7H,1-2H3,(H,23,26)(H,21,27,28)
|
|
| 化学名 |
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 3.75 mg/mL (8.62 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 37.5 mg/mL 澄清的 DMSO 储备液加入到400 μL PEG300中,混匀;再向上述溶液中加入50 μL Tween-80,混匀;然后加入450 μL 生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 3.75 mg/mL (8.62 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 37.5 mg/mL 澄清 DMSO 储备液加入 900 μL 20% SBE-β-CD 生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 3.75 mg/mL (8.62 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.2977 mL | 11.4884 mL | 22.9769 mL | |
| 5 mM | 0.4595 mL | 2.2977 mL | 4.5954 mL | |
| 10 mM | 0.2298 mL | 1.1488 mL | 2.2977 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Model of53(MGL-3196, magenta) bound to THR-β (1N46) with the T3 geometry (cyan) from3GWSsuperimposed. Polar interactions of53in the anion binding site are highlighted.J Med Chem.2014 May 22;57(10):3912-23. |
|---|
(A) 2D description of the binding site for T3 (PDB code3GWS). (B) 2D description of the binding site for the53model (MOE).J Med Chem.2014 May 22;57(10):3912-23. |
Left panel: cardiac α-MHC hnRNA relative levels (arbitrary units) in untreated thyroidectomized rats (control), euthyroid rats, and thyroidectomized rats 6 h after exposure to53dosed intraperitoneally at the specified doses.Right panel: activities of tested compounds relative to full activity (euthyroid or T3-treated) and exposure of the compound 6 h after dose.J Med Chem.2014 May 22;57(10):3912-23. |
Effects of53(MGL-3196) vs T3 on cholesterol and BMD in DIO mice.J Med Chem.2014 May 22;57(10):3912-23. |
|---|