| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg | |||
| Other Sizes |
| 靶点 |
M1 ( Ki = 0.42 nM ); M2 ( Ki = 0.32 nM ); M3 ( Ki = 0.18 nM ); M4 ( Ki = 0.56 nM ); M5 ( Ki = 6.7 nM )
|
|---|---|
| 体外研究 (In Vitro) |
Revefenacin 对人 M1、M2、M3、M4 和 M5 受体的 Ki 值分别为 0.42、0.32、0.18、0.56 和 6.7 nM。在功能测定中,revefenacin 被证明是一种功能性拮抗剂,其抑制常数与结合 Kis 相似。 Revefenacin 还可以抑制激动剂诱导的豚鼠离体气管环制剂的收缩,亲和力为 0.1 nM,与测量的 M3 结合 Ki 相似[1]。
|
| 体内研究 (In Vivo) |
在麻醉犬中,瑞芬那新与噻托溴铵和格隆溴铵一起,对乙酰胆碱诱导的支气管收缩产生长达 24 小时的持续抑制。在麻醉大鼠中,吸入瑞芬那新对乙酰甲胆碱诱导的支气管收缩表现出剂量依赖性的 24 小时支气管保护作用。估计的 24 小时效力为 45.0 µg/mL,每日一次给药 7 天后仍能保持支气管保护效力[2]。
|
| 动物实验 |
Rats: Rats are exposed by inhaling a nebulized solution of either vehicle (sterile water) or revefenacin (3–3000 µg/mL), tiotropium (0.3–300 µg/mL), or glycopyrronium (1–1000 µg/mL) to ascertain the bronchoprotective and antisialagogue potency after a single dose. 24 hours after the dosage, bronchoprotective activity is evaluated. The antisialagogue effect's peak effect time is determined by measuring the inhibition of Pilo 1, 6, or 12 hours after an effective dose of the test compound was inhaled. At this point in time, all subsequent doses are measured[2].
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
In pharmacokinetic studies, revefenacin was absorbed very rapidly and presented a linear increase in plasma exposure with Cmax, tmax and AUC that ranged between 0.02-0.15 ng/ml, 0.48-0.51 hours and 0.03-0.36 ng.h/ml, respectively. The bioaccumulation of revefenacin was very limited and the steady-state was achieved by day 7. After reaching maximum concentration, revefenacin concentrations decline in a biphasic manner. This elimination kinetics is observed by a rapid declining plasma concentration followed by a slow apparent bi-exponential elimination. Renal elimination of revefenacin is limited and it presents a mean cumulative amount excreted in urine as the unchanged drug of < 0.2% of the administered dose. Following intravenous revefenacin administration, 54% of the dose is recovered in feces and 27% was recovered in urine which confirms a high hepatobiliary processing. After intravenous administration of revefenacin, the reported volume of distribution is 218 L which suggests an extensive distribution to the tissues. The renal clearance of revefenacin is negligible and thus, the clearance rate is not a major parameter for this drug. Metabolism / Metabolites Revefenacin presents a high metabolic liability producing a rapid metabolic turnover after being distributed from the lung. This metabolic process is done primarily via enzymatic hydrolysis via CYP2D6 to its major hydrolytic metabolite THRX-195518. Biological Half-Life The apparent terminal half-life of a dose of 350 mcg of revefenacin was 22.3-70 hours. |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
Like other anticholinergic agents, revefenacin has not been linked to episodes of liver enzyme elevations or clinically apparent liver injury. Another reason for its hepatic safety may relate to its low systemic absorption when administered by inhaler. Likelihood score: E (unlikely cause of clinically apparent liver injury). Drug Class: Anticholinergic Agents Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation No information is available on the use of revefenacin during breastfeeding. Because the drug is only 3% absorbed orally, it is unlikely to affect the breastfed infant. Long-term use of revefenacin might reduce milk production or milk letdown. During long-term use, observe for signs of decreased lactation (e.g., insatiety, poor weight gain). ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding The protein binding of revefenacin and its active metabolite is of 71% and 42% respectively. |
| 参考文献 |
|
| 其他信息 |
Revefenacin is a novel biphenyl carbamate tertiary amine agent that belongs to the family of the long-acting muscarinic antagonists (LAMA). The labile primary amide in the structure produces a "soft-drug" site that allows rapid systemic clearance and minimizing of the systemically mediated adverse reactions. The LAMA group falls into a parent category known as long-acting inhaled bronchodilators and this type of agents are recommended as a maintenance therapy for chronic obstructive pulmonary disease (COPD). From the LAMA group, revefenacin is the first once-daily nebulized LAMA treatment. It was developed by Theravance Biopharma and FDA approved on November 9, 2018.
Revefenacin is an Anticholinergic. The mechanism of action of revefenacin is as a Cholinergic Antagonist. Revefenacin is a synthetic anticholinergic agent that is used as a once daily, nebulized inhalant for maintenance treatment of patients with chronic obstructive pulmonary disease. Revefenacin has not been implicated in causing liver enzyme elevations or clinically apparent acute liver injury. Drug Indication Revefenacin is indicated as an inhalation solution for the maintenance treatment of patients with chronic obstructive pulmonary disease (COPD). COPD is a growing disease being the third leading cause of death in the US. This disease is characterized by not fully reversible airflow limitation. FDA Label Mechanism of Action Revefenacin is an inhaled bronchodilator muscarinic antagonist with a long-acting bronchodilation activity. It has been shown to present a high affinity and behaved as a competitive antagonist of the five muscarinic cholinergic receptors. Studies have indicated that revefenacin dissociates significantly slower from the muscarinic receptor M3 (hM3) when compared to the receptor M2 (hM2) which indicates a kinetic selectivity for this subtype. This competitive antagonism produces a suppressive action of the acetylcholine-evoked calcium mobilization and contractile responses in the airway tissue. Lastly, due to the duration of the bronchodilation, revefenacin is considered a long-acting muscarinic antagonist which allows it to be dosed once daily. This response is very important for the therapy of COPD as the main goal is the reduce the frequency and severity of exacerbations which are normally driven by the presence of elevated cholinergic bronchoconstrictor tone mediated by muscarinic receptors on parasympathetic ganglia and airway smooth muscle. Hence, the activity of revefenacin produces a potent and long-lasting protection against the bronchoconstrictor response to acetylcholine or methacholine. Pharmacodynamics Revefenacin has been reported to produce a sustained, long-acting bronchodilation with lower anti-muscarinic-related side effects. In clinical trials, revefenacin demonstrated to be of a long duration of action and low systemic exposure in patients with COPD. Also, it was reported that a dose of 88 mcg can produce a clinically effective bronchodilation measured by through forced expiratory volume in 1s and serial spirometric assessments. In placebo-controlled trials, revefenacin showed a decrease in the use of albuterol rescue inhalers and sustained increases in the peak expiratory flow rate that reached a steady state at a maximum in day 7. As well, there was a reported superior lung selectivity index when compared with other LAMAs such as glycopyrronium and tiotropium which produced a decreased sialagogue effect. |
| 分子式 |
C35H43N5O4
|
|---|---|
| 分子量 |
597.76
|
| 精确质量 |
597.331
|
| 元素分析 |
C, 70.33; H, 7.25; N, 11.72; O, 10.71
|
| CAS号 |
864750-70-9
|
| 相关CAS号 |
864750-70-9; 864751-51-9 (phosphate); 864751-53-1 (sulfate); 864751-55-3 (oxalate)
|
| PubChem CID |
11753673
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.3±0.1 g/cm3
|
| 沸点 |
777.5±60.0 °C at 760 mmHg
|
| 闪点 |
424.0±32.9 °C
|
| 蒸汽压 |
0.0±2.7 mmHg at 25°C
|
| 折射率 |
1.645
|
| LogP |
3.22
|
| tPSA |
108
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
6
|
| 可旋转键数目(RBC) |
11
|
| 重原子数目 |
44
|
| 分子复杂度/Complexity |
918
|
| 定义原子立体中心数目 |
0
|
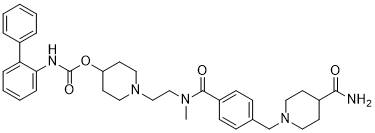
| SMILES |
O=C(NC1C(C2C=CC=CC=2)=CC=CC=1)OC1CCN(CCN(C)C(C2C=CC(CN3CCC(C(N)=O)CC3)=CC=2)=O)CC1
|
| InChi Key |
FYDWDCIFZSGNBU-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C35H43N5O4/c1-38(34(42)29-13-11-26(12-14-29)25-40-19-15-28(16-20-40)33(36)41)23-24-39-21-17-30(18-22-39)44-35(43)37-32-10-6-5-9-31(32)27-7-3-2-4-8-27/h2-14,28,30H,15-25H2,1H3,(H2,36,41)(H,37,43)
|
| 化学名 |
[1-[2-[[4-[(4-carbamoylpiperidin-1-yl)methyl]benzoyl]-methylamino]ethyl]piperidin-4-yl] N-(2-phenylphenyl)carbamate
|
| 别名 |
TD-4208; TD4208; GSK-1160724; GSK-1160724; TD 4208; GSK1160724; trade name: Yupelri; TD-4208; GSK 1160724
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (4.18 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (4.18 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (4.18 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.6729 mL | 8.3646 mL | 16.7291 mL | |
| 5 mM | 0.3346 mL | 1.6729 mL | 3.3458 mL | |
| 10 mM | 0.1673 mL | 0.8365 mL | 1.6729 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT04315558 | Recruiting | Drug: Ipratropium Bromide Drug: Revefenacin Inhalation Solution [Yupelri] |
COPD Acute Respiratory Failure |
University of California, Los Angeles |
November 1, 2020 | Phase 2 |
| NCT04655170 | Recruiting | Drug: Revefenacin (YUPELRI) & Formoterol (Perforomist) |
COPD Exacerbation | University of Tennessee Graduate School of Medicine |
December 9, 2020 | Phase 4 |
| NCT03573817 | Completed | Drug: Revefenacin Drug: Placebo |
Chronic Obstructive Pulmonary Disease (COPD) |
Mylan Inc. | May 31, 2018 | Phase 3 |
| NCT05165485 | Completed | Drug: Revefenacin Drug: Tiotropium |
Chronic Obstructive Pulmonary Disease (COPD) |
Theravance Biopharma | January 7, 2022 | Phase 4 |
| NCT03095456 | Recruiting | Drug: Revefenacin Drug: Placebo for Revefenacin |
Low Peak Inspiratory Flow Rate (PIFR) |
Mylan Inc. | March 27, 2017 | Phase 3 |