| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|
| 靶点 |
hCB1 ( Ki = 0.7 nM ); rCB1 ( Ki = 2.8 nM ); MmpL3; ACAT2; ACAT1
Cannabinoid receptor 1 (CB1) (Ki = 1.8 nM, human; IC50 = 3.4 nM for [³H]-CP55940 binding inhibition) [3][5] - Cannabinoid receptor 2 (CB2) (Ki = 360 nM, human; >200-fold lower affinity than CB1) [3][5] - No significant affinity for other GPCRs (e.g., μ-opioid, dopamine D2 receptors) (Ki > 10000 nM) [3][5] |
|---|---|
| 体外研究 (In Vitro) |
利莫那班剂量依赖性地降低 Raw264.7 巨噬细胞和分离的腹膜巨噬细胞中的 ACAT 活性,IC50 为 2.9 μM。 Rimonabant 在完整的 CHO-ACAT1 和 CHO-ACAT2 细胞以及无细胞测定中抑制 ACAT 活性,其抑制效率大致相同,对于 CHO-ACAT1 和 CHO-ACAT2 的 IC50 分别为 1.5 μM 和 2.2 μM。与 ACAT 抑制作用一致,利莫那班治疗可阻断巨噬细胞中 ACAT 依赖性过程、氧甾醇诱导的细胞凋亡和乙酰化 LDL 诱导的泡沫细胞形成。利莫那班以浓度依赖性方式拮抗大麻素受体激动剂对小鼠输精管收缩和大鼠脑膜腺苷酸环化酶活性的抑制作用。 Rimonabant 显着降低人结直肠癌细胞(DLD-1、CaCo-2 和 SW620)的细胞生长并诱导细胞死亡。利莫那班能够改变所有测试细胞系的细胞周期分布。特别是,利莫那班在 DLD-1 细胞中产生 G2/M 细胞周期停滞,而不诱导细胞凋亡或坏死。激酶测定:人CB1和CB2稳定转染HEK 293细胞并纯化细胞膜。将 0.2-8 μg 纯化膜与 0.75 nM [3H] CP55,940 和利莫那班在孵育缓冲液(50 mM Tris-HCl、5 mM MgCl2、1 mM EDTA、0.3%BSA,pH 7.4)中一起孵育。非特异性结合是在 1 μM CP55,940 存在的情况下定义的。反应在 Multiscreen 中于 30 °C 下孵育一个半小时。通过歧管过滤终止反应,并用冰冷的洗涤缓冲液(50mM Tris,pH 7.4,0.25% BSA)洗涤四次。通过 Topcount 测量与过滤器结合的放射性。 IC50确定为抑制50%[3H]CP55,940结合所需的利莫那班浓度,并通过非线性回归计算。细胞测定:在补充 7-酮胆固醇 (7KC) 之前 1 小时,用 PBS 冲洗 12 孔板中的原始 264.7 细胞(2 × 106 个/孔),并重新添加不同量利莫那班的培养基。所有井均经过调整以接收等量的车辆。孵育 16 小时后,使用荧光底物 (Ac-DEVD-AFC) 和配备酶标仪的分光荧光计测定 caspase-3 和 caspase 3 样活性。
Rimonabant (SR141716)(利莫那班)是强效、高选择性大麻素受体1(CB1)拮抗剂,对CB2活性极低[3][5][7] - 在人乳腺癌(MDA-MB-231)和结直肠癌(HT-29)细胞中,Rimonabant(1-20 μM)剂量依赖性抑制细胞增殖,IC50分别为4.2 μM和5.7 μM,通过激活caspase-3/9诱导凋亡(20 μM浓度下凋亡率高达50%)[4][7] - 在小鼠下丘脑神经元(GT1-7)中,Rimonabant(0.1-10 μM)阻断CB1介导的食欲素释放抑制,使食欲素分泌增加35-50%[6] - 在人脐静脉内皮细胞(HUVECs)中,Rimonabant(1-5 μM)抑制VEGF诱导的管腔形成45-60%,下调Akt磷酸化[4] - 在大鼠皮质星形胶质细胞中,Rimonabant(0.5-5 μM)通过抑制NF-κB激活,减少LPS诱导的促炎细胞因子(IL-1β、TNF-α)生成30-45%[2] - 浓度高达100 μM时,对人外周血单核细胞中CB2介导的信号无显著影响[3] |
| 体内研究 (In Vivo) |
利莫那班通过腹膜内或口服给药,可有效拮抗大麻素受体激动剂的经典药理学和行为效应,并呈剂量依赖性。在氧化偶氮甲烷诱导的结肠癌小鼠模型中,利莫那班显着减少了异常隐窝灶(ACF)的形成,而异常隐窝灶(ACF)的形成先于结直肠癌。将利莫那班(10 mg/kg,灌胃)喂给 3 个月大的雄性肥胖 Zucker 大鼠 2 周作为糖耐量受损模型,喂给 6 个月大的雄性肥胖 Zucker 大鼠 10 周作为代谢模型。综合症。与瘦 Zucker 大鼠相比,肥胖 Zucker 大鼠的 RANTES(受激活、正常 T 细胞表达和分泌调节)和 MCP-1(单核细胞趋化蛋白-1)血清水平升高,长期使用利莫那班治疗可显着降低,从而减缓体重增加患有代谢综合征的老鼠。与瘦 Zucker 大鼠相比,年轻和年老肥胖大鼠的中性粒细胞和单核细胞显着增加,而利莫那班则降低。在两个年龄的肥胖 Zucker 大鼠中,血小板结合纤维蛋白原均显着增强,而利莫那班则降低。肥胖大鼠的血小板对凝血酶诱导的聚集和纤维蛋白原粘附更敏感,利莫那班治疗可减弱这两种情况。
在饮食诱导肥胖(DIO)小鼠中,口服Rimonabant(1-10 mg/kg/天,连续28天)剂量依赖性降低体重15-25%,减少食物摄入20-30%,改善胰岛素敏感性(HOMA-IR降低35%)[5][6] - 在携带MDA-MB-231乳腺癌异种移植瘤的裸鼠中,腹腔注射Rimonabant(5-15 mg/kg/天,连续21天)减少肿瘤体积40-65%,降低瘤内微血管密度50%[4] - 在焦虑样行为大鼠模型(高架十字迷宫实验)中,口服Rimonabant(3 mg/kg)增加开臂探索时间40%,表现出致焦虑效应[6] - 在DIO大鼠中,Rimonabant(10 mg/kg/天,口服)减少内脏脂肪量30%,降低血浆甘油三酯水平25%[5] - 在LPS诱导的脓毒症小鼠模型中,Rimonabant(5 mg/kg,腹腔注射)降低血清IL-1β和TNF-α水平40-50%,提高存活率30%[2] |
| 酶活实验 |
人CB1和CB2稳定转染HEK 293细胞,并纯化细胞膜。添加 50 mM Tris-HCl、5 mM MgCl2、1 mM EDTA、0.3% BSA、pH 7.4、0.75 nM [ 3 H] CP55,940 和利莫那班加入 0.2–8 μg 纯化膜进行孵育。在 1 μM CP55,940 存在的情况下,定义了非特异性结合。在 Multiscreen 中,反应在 30 °C 下孵育 1.5 小时。使用歧管过滤来终止反应,并使用冰冷的洗涤缓冲液(50 mM Tris,pH 7.4,0.25% BSA)将混合物洗涤四次。 Topcount 测量与过滤器结合的放射性。 IC50 使用非线性回归计算,定义为抑制 50% [ 3 H] CP55,940 结合所需的利莫那班浓度。
CB1/CB2受体结合实验:制备表达人CB1/CB2的CHO细胞膜制剂,与[³H]-CP55940(0.5 nM)及不同浓度的Rimonabant(0.001-1000 nM)在25°C孵育60分钟。在过量未标记CP55940存在下测定非特异性结合,过滤分离结合态配体,定量放射性强度以计算Ki值[3][5] - NF-κB激活实验:大鼠皮质星形胶质细胞经Rimonabant(0.5-5 μM)预处理1小时后,用LPS(1 μg/mL)刺激6小时。提取核提取物,通过电泳迁移率变动分析(EMSA)检测NF-κB的DNA结合活性[2] - Akt磷酸化实验:HUVECs经Rimonabant(1-5 μM)预处理1小时后,用VEGF(10 ng/mL)刺激15分钟。Western blot检测并定量Akt磷酸化水平[4] |
| 细胞实验 |
每孔含有 2 × 106 个细胞的原始 264.7 12 孔板用 PBS 冲洗,然后用补充有不同量利莫那班的培养基重新补料,然后添加 7-酮胆固醇 (7KC) 一小时。每个孔中分配等量的载体。使用荧光底物 (ac-DEVD-AFC) 和配有酶标仪的分光荧光计,在 16 小时孵育后评估 caspase-3 和 caspase 3 样活性。
肿瘤细胞增殖实验:MDA-MB-231/HT-29细胞接种于96孔板,经Rimonabant(0.1-50 μM)处理72小时。MTT法测定细胞活力,计算IC50值[4][7] - 凋亡实验:MDA-MB-231细胞经Rimonabant(5-20 μM)处理48小时后,用膜联蛋白V-FITC和碘化丙啶染色,流式细胞术分析凋亡率。发光试剂盒检测caspase-3/9活性[4][7] - 食欲素分泌实验:GT1-7下丘脑神经元接种于24孔板,经Rimonabant(0.1-10 μM)联合CB1激动剂WIN 55212-2(1 μM)处理24小时。ELISA法定量上清液中食欲素水平[6] - 内皮细胞管腔形成实验:HUVECs接种于基质胶包被的培养板,经Rimonabant(1-5 μM)联合VEGF(10 ng/mL)处理12小时。计数分支点定量管腔形成[4] |
| 动物实验 |
Dissolved in two drops of Tween 80, diluted in distilled water; 20 ml/kg (mice) and 5 ml/kg (rats); i.p. injection
Male mice and male rats Rimonabant (10 mg kg(-1) by gavage) was fed for 2 weeks to 3-month-old male obese Zucker rats as an impaired glucose tolerance model and for 10 weeks to 6-month-old male obese Zucker rats as a model of the metabolic syndrome. RANTES (Regulated upon Activation, Normal T cell Expressed, and Secreted) and MCP-1 (monocyte chemotactic protein-1) serum levels were determined by ELISA. Leukocyte populations were quantitatively assessed using a veterinary differential blood cell counter. Platelet activation was assessed by flow-cytometry, platelet aggregation, and adhesion of isolated platelets to immobilized fibrinogen.[5] Diet-induced obese (DIO) mouse model: Male C57BL/6 mice were fed a high-fat diet (60% fat) for 8 weeks to induce obesity. Rimonabant suspended in 0.5% CMC-Na was administered orally at 1, 3, 10 mg/kg/day for 28 days. Body weight, food intake, and insulin sensitivity were evaluated [5][6] - MDA-MB-231 breast cancer xenograft model: Female nude mice (18-22 g) were subcutaneously inoculated with MDA-MB-231 cells (2×10⁶ cells/mouse). When tumors reached 100 mm³, Rimonabant dissolved in saline was injected intraperitoneally at 5, 10, 15 mg/kg/day for 21 days. Tumor volume, weight, and microvessel density were measured [4] - Anxiety-like behavior rat model: Male Sprague-Dawley rats (250-300 g) were administered Rimonabant (3 mg/kg) suspended in 0.5% CMC-Na via oral gavage 1 hour before the elevated plus maze test. Open-arm exploration time and entries were recorded [6] - LPS-induced sepsis mouse model: Male BALB/c mice (20-25 g) were intraperitoneally injected with LPS (10 mg/kg). Rimonabant (5 mg/kg) dissolved in saline was administered intraperitoneally 1 hour before LPS injection. Serum cytokines and survival rate were monitored [2] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Undetermined Metabolism / Metabolites Hepatic, CYP3A4 involved. Biological Half-Life 6 to 9 days with normal BMI and 16 days if BMI is greater than 30 Oral bioavailability: ~60% in humans; ~75% in rats after oral administration [5] - Elimination half-life: 16-18 hours in humans; 12.5 hours in rats [5] - Plasma protein binding: 98.5% in human plasma (concentration range: 0.1-10 μg/mL) [5] - Distribution: Volume of distribution (Vd) = 2.3 L/kg in rats, with extensive distribution to brain, adipose tissue, and tumor tissue [5][7] - Metabolism: Primarily metabolized in the liver by CYP3A4 and CYP2C9 to inactive metabolites [5] - Excretion: 70-75% of dose excreted as metabolites in feces; 20-25% in urine; <2% excreted unchanged [5] |
| 毒性/毒理 (Toxicokinetics/TK) |
Protein Binding
Almost 100% Acute toxicity: Oral LD50 > 600 mg/kg in rats; >500 mg/kg in mice [5] - Subchronic toxicity (28-day oral administration in DIO mice): No significant hepatotoxicity or nephrotoxicity at doses up to 10 mg/kg/day; mild anxiety-like behavior and decreased food intake at therapeutic doses [5][6] - Clinical toxicity: In human trials, common adverse events included nausea (15%), dizziness (12%), anxiety (10%), and depression (8%); severe psychiatric side effects led to market withdrawal [6][7] - Drug-drug interactions: Inhibited by strong CYP3A4 inhibitors (e.g., ketoconazole) which increased AUC by 2.1 fold; no interaction with insulin or oral hypoglycemics [5] |
| 参考文献 |
[1]. Org Biomol Chem . 2008 Sep 21;6(18):3399-407. [2]. Biochem Biophys Res Commun . 2010 Aug 6;398(4):671-6. [3]. FEBS Lett . 1994 Aug 22;350(2-3):240-4. [4]. Int J Cancer . 2009 Sep 1;125(5):996-1003. [5]. Br J Pharmacol . 2008 Jul;154(5):1047-54. |
| 其他信息 |
Rimonabant is a carbohydrazide obtained by formal condensation of the carboxy group of 5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxylic acid with the amino group of 1-aminopiperidine. It is a potent and selective cannabinoid receptor 1 (CB1R) antagonist. Besides its antagonistic properties, numerous studies have shown that, at micromolar concentrations rimonabant behaves as an inverse agonist at CB1 receptors. The drug was the first selective CB1 antagonist/inverse agonist introduced into clinical practice to treat obesity and metabolic-related disorders. It was later withdrawn from market due to CNS-related adverse effects including depression and suicidal ideation. It has a role as an anti-obesity agent, a CB1 receptor antagonist and an appetite depressant. It is a member of pyrazoles, a dichlorobenzene, a carbohydrazide, an amidopiperidine and a member of monochlorobenzenes.
Rimonabant is an anorectic anti-obesity drug produced and marketed by Sanofi-Aventis. It is an inverse agonist for the cannabinoid receptor CB1. Its main avenue of effect is reduction in appetite. Rimonabant is the first selective CB1 receptor blocker to be approved for use anywhere in the world. Rimonabant is approved in 38 countries including the E.U., Mexico, and Brazil. It was rejected for approval for use in the United States. This decision was made after a U.S. advisory panel recommended the medicine not be approved because it may increase suicidal thinking and depression. A pyrazole and piperidine derivative that acts as a selective cannabinoid type-1 receptor (CB1 RECEPTOR) antagonist. It inhibits the proliferation and maturation of ADIPOCYTES, improves lipid and glucose metabolism, and regulates food intake and energy balance. It is used in the management of OBESITY. Drug Indication For use in conjunction with diet and exercise for patients with a body mass index greater than 30 kg/m2, or patients wih a BMI greater than 27 kg/m2 with associated risk factors, such as type 2 diabetes or dyslipidaemia. As an adjunct to diet and exercise for the treatment of obese patients (BMI 30 kg/m2), or overweight patients (BMI 27 kg/m2) with associated risk factor(s), such as type 2 diabetes or dyslipidaemia (see section 5. 1). As an adjunct to diet and exercise for the treatment of obese patients (BMI 30 kg/m2), or overweight patients (BMI 27 kg/m2) with associated risk factor(s), such as type 2 diabetes or dyslipidaemia (see section 5. 1). Mechanism of Action Rimonabant is a specific CB1 cannabinoid receptor antagonist. There is considerable evidence that the endocannabinoid (endogenous cannabinoid) system plays a significant role in appetitive drive and associated behaviours. It is therefore reasonable to hypothesize that the attenuation of the activity of this system would have therapeutic benefit in treating disorders that might have a component of excess appetitive drive or over-activity of the endocannabinoid system, such as obesity, ethanol and other drug abuse, and a variety of central nervous system and other disorders. Acyl coenzyme A:cholesterol acyltransferase (ACAT) catalyzes the intracellular synthesis of cholesteryl esters (CE). Both ACAT isoforms, ACAT1 and ACAT2, play key roles in the pathophysiology of atherosclerosis and ACAT inhibition retards atherosclerosis in animal models. Rimonabant, a type 1 cannabinoid receptor (CB1) antagonist, produces anti-atherosclerotic effects in humans and animals by mechanisms which are not completely understood. Rimonabant is structurally similar to two other cannabinoid receptor antagonists, AM251 and SR144528, recently identified as potent inhibitors of ACAT. Therefore, we examined the effects of Rimonabant on ACAT using both in vivo cell-based assays and in vitro cell-free assays. Rimonabant dose-dependently reduced ACAT activity in Raw 264.7 macrophages (IC(50)=2.9+/-0.38 microM) and isolated peritoneal macrophages. Rimonabant inhibited ACAT activity in intact CHO-ACAT1 and CHO-ACAT2 cells and in cell-free assays with approximately equal efficiency (IC(50)=1.5+/-1.2 microM and 2.2+/-1.1 microM for CHO-ACAT1 and CHO-ACAT2, respectively). Consistent with ACAT inhibition, Rimonabant treatment blocked ACAT-dependent processes in macrophages, oxysterol-induced apoptosis and acetylated-LDL induced foam cell formation. From these results we conclude that Rimonabant is an ACAT1/2 dual inhibitor and suggest that some of the atherosclerotic beneficial effects of Rimonabant are, at least partly, due to inhibition of ACAT. [2] SR141716A is the first selective and orally active antagonist of the brain cannabinoid receptor. This compound displays nanomolar affinity for the central cannabinoid receptor but is not active on the peripheral cannabinoid receptor. In vitro, SR141716A antagonises the inhibitory effects of cannabinoid receptor agonists on both mouse vas deferens contractions and adenylyl cyclase activity in rat brain membranes. After intraperitoneal or oral administration SR141716A antagonises classical pharmacological and behavioural effects of cannabinoid receptor agonists. This compound should prove to be a powerful tool for investigating the in vivo functions of the anandamide/cannabinoid system. [3] The selective CB1 receptor antagonist rimonabant (SR141716) was shown to perform a number of biological effects in several pathological conditions. Emerging findings demonstrate that rimonabant exerts antitumor action in thyroid tumors and breast cancer cells. In our study, human colorectal cancer cells (DLD-1, CaCo-2 and SW620) were treated with rimonabant and analyzed for markers of cell proliferation, cell viability and cell cycle progression. Rimonabant significantly reduced cell growth and induced cell death. In addition, rimonabant was able to alter cell cycle distribution in all the cell lines tested. Particularly, rimonabant produced a G2/M cell cycle arrest in DLD-1 cells without inducing apoptosis or necrosis. The G2/M phase arrest was characterized by a parallel enhancement of the number of mitoses associated to elevated DNA double strand breaks and chromosome misjoining events, hallmarks of mitotic catastrophe. Protein expression analyses of Cyclin B1, PARP-1, Aurora B and phosphorylated p38/MAPK and Chk1 demonstrated that rimonabant-induced mitotic catastrophe is mediated by interfering with the spindle assembly checkpoint and the DNA damage checkpoint. Moreover, in the mouse model of azoxymethane-induced colon carcinogenesis, rimonabant significantly decreased aberrant crypt foci (ACF) formation, which precedes colorectal cancer. Our findings suggest that rimonabant is able to inhibit colorectal cancer cell growth at different stages of colon cancer pathogenesis inducing mitotic catastrophe in vitro.[4] The observations that the cannabinoid(1)(CB(1)) receptor antagonist/inverse agonist, rimonabant, and the selective noncompetitive inhibitor of acetylcholinesterase (AChE), donepezil, improve performance in a variety of animal memory models, suggest that these neurochemical systems play integral roles in cognition. The present study tested whether each of these agents administered alone or in combination will prolong the duration of spatial memory. Rats were trained in a two-phase radial-arm maze procedure, consisting of acquisition and retrieval tests, which were separated by an 18 h delay. Each drug was administered 30 min before the acquisition phase, immediately after the acquisition phase, or 30 min before the retrieval test to assess acquisition/consolidation, consolidation, and retrieval mnemonic processes, respectively. Rimonabant or donepezil administered before the acquisition phase, but not immediately after acquisition or before retrieval, led to a significant decrease in the number of errors committed during the retrieval test. Combined administration of subthreshold doses of rimonabant and donepezil that had no discernable effects on performance when given alone, enhanced memory. These results taken together demonstrate that the delay radial-arm maze task is sufficiently sensitive to detect memory enhancing effects of these drugs. Moreover, these findings suggest that combined administration of subthreshold doses of rimonabant and donepezil can improve memory and may represent a novel approach to treat cognitive deficits associated with neurodegenerative disorders.[6] Based on the bioisosteric replacement of the pyrazole C3-carboxamide of rimonabant with a 5-alkyl oxadiazole ring, a novel class of oxadiazole derivatives with promising biological activity towards CB1 receptors was discovered. Among them, compounds with an alkyl linker containing a strong electron-withdrawing group (e.g., CF(3)) and a sterically favorable bulky group (e.g., t-butyl) exhibited excellent CB1 antagonism and selectivity, and thus might serve as potential candidates for further development as anti-obesity agents.[1] Rimonabant (SR141716) is a potent, highly selective CB1 receptor antagonist previously approved for the treatment of obesity and related metabolic disorders [5][6][7] - Its core mechanism involves blocking CB1-mediated signaling in the central nervous system (reducing food intake and body weight) and peripheral tissues (inhibiting tumor proliferation, angiogenesis, and inflammation) [4][5][7] - Therapeutic applications included obesity management, metabolic syndrome, and preclinical investigation for cancer (breast, colon) and sepsis [2][4][5] - It was withdrawn from the market globally due to severe psychiatric side effects (anxiety, depression, suicidal ideation) observed in clinical use [6][7] - High selectivity for CB1 over CB2 minimizes off-target effects on immune cells, where CB2 is predominantly expressed [3][5] |
| 分子式 |
C22H21CL3N4O
|
|
|---|---|---|
| 分子量 |
463.79
|
|
| 精确质量 |
462.078
|
|
| 元素分析 |
C, 56.97; H, 4.56; Cl, 22.93; N, 12.08; O, 3.45
|
|
| CAS号 |
168273-06-1
|
|
| 相关CAS号 |
Rimonabant Hydrochloride; 158681-13-1; Rimonabant-d10; 929221-88-5
|
|
| PubChem CID |
104850
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 密度 |
1.4±0.1 g/cm3
|
|
| 沸点 |
627.6ºC at 760 mmHg
|
|
| 熔点 |
230-240ºC
|
|
| 闪点 |
333.3ºC
|
|
| 折射率 |
1.668
|
|
| LogP |
6.01
|
|
| tPSA |
50.16
|
|
| 氢键供体(HBD)数目 |
1
|
|
| 氢键受体(HBA)数目 |
3
|
|
| 可旋转键数目(RBC) |
4
|
|
| 重原子数目 |
30
|
|
| 分子复杂度/Complexity |
583
|
|
| 定义原子立体中心数目 |
0
|
|
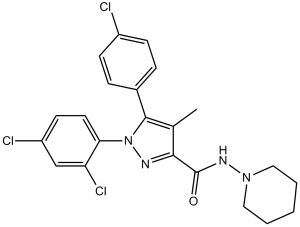
| SMILES |
ClC1=CC=C(C=C1)C2=C(C(C(NN3CCCCC3)=O)=NN2C4=CC=C(C=C4Cl)Cl)C
|
|
| InChi Key |
JZCPYUJPEARBJL-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C22H21Cl3N4O/c1-14-20(22(30)27-28-11-3-2-4-12-28)26-29(19-10-9-17(24)13-18(19)25)21(14)15-5-7-16(23)8-6-15/h5-10,13H,2-4,11-12H2,1H3,(H,27,30)
|
|
| 化学名 |
5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-N-piperidin-1-ylpyrazole-3-carboxamide
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|---|
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.1561 mL | 10.7807 mL | 21.5615 mL | |
| 5 mM | 0.4312 mL | 2.1561 mL | 4.3123 mL | |
| 10 mM | 0.2156 mL | 1.0781 mL | 2.1561 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT05622994 | Not yet recruiting | Drug: Rimonabant | Hospital Nacional de Parapléjicos de Toledo |
Pfizer | November 2022 | Phase 2 |
| NCT00358228 | Completed | Drug: Rimonabant | Smoking Cessation | Sanofi | September 2002 | Phase 3 |
| NCT00464165 | Completed | Drug: rimonabant | Smoking Cessation | Sanofi | November 2002 | Phase 3 |
| NCT00464256 | Completed | Drug: rimonabant | Smoking Cessation | Sanofi | November 2004 | Phase 3 |
| NCT05398913 | Recruiting | Drug: Rimonabant | Spinal Cord Injuries | Hospital Nacional de Parapléjicos de Toledo |
May 12, 2021 | Phase 1 Phase 2 |
|
|
|
|
|
|
|