| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
PKC-α (IC50 = 5 nM); PKC-βII (IC50 = 14 nM); MAPKAP-K1b (IC50 = 3 nM); MSK1 (IC50 = 8 nM); S6K1 (IC50 = 15 nM);PKC-βI (IC50 = 24 nM); PKC-ε (IC50 = 24 nM); PKC-γ (IC50 = 27 nM); Rat Brain PKC (IC50 = 23 nM); GSK3β (IC50 = 38 nM)
|
||
|---|---|---|---|
| 体外研究 (In Vitro) |
Ro 31-8220 是一种强效 PKC 抑制剂,对 PKCα、PKCβI、PKCβII、PKCγ、PKCε 和大鼠脑 PKC 的 IC50 值分别为 5、24、14、27、24 和 23 nM[1]。此外,Ro 31-8220 强烈抑制 MSK1、S6K1、GSK3β 和 MAPKAP-K1b(IC50 分别为 3、8、15 和 38 nM); MKK3、MKK4、MKK6 和 MKK7 不受影响。此外,电压依赖性 Na+ 通道直接被 Ro 31-8220[2] 抑制。在小脑颗粒神经元中,Ro 31-8220 (1 μM) 对对氧磷诱导的神经元细胞死亡具有神经保护作用,抑制对氧磷诱导的 caspase-3 活性,并降低对氧磷引起的磷酸 PKC pan 水平的增加 [3]。
|
||
| 体内研究 (In Vivo) |
在小鼠中,Ro 31-8220(6 mg/kg/d,皮下注射)的半衰期为 5.7 小时,且耐受性良好。经过六周的治疗后,用 Ro 31-8220 治疗的 MLP−/− 小鼠在缩短分数方面表现出显着的改善,但 WT 小鼠没有表现出任何变化[4]。
药理抑制PKCα可恢复MLP−/−小鼠的心功能[4] 由于传统的肌力药物慢性治疗与心力衰竭患者的不良结果相关,因此我们在这里研究了慢性给予Ro-31-8220超过4-6周对MLP - / -心力衰竭小鼠的影响。在研究开始时和6周后,通过超声心动图评估所有小鼠的心室功能。Ro-31-8220(或对照品)每天注射一次,剂量为6mg /kg/day, s.q。Ro-31-8220化合物被用于Ro-32-0432化合物的所有体内研究,只是因为与大规模合成相关的费用问题以及在小鼠中长期使用所需的量。Ro-31-8220在大鼠体内的药动学分析 药物的半衰期为5.7小时,6小时时血浆浓度比PKCα的IC50高100倍(见讨论)。该剂量的Ro-31-8220在小鼠中耐受性良好,在6周内没有观察到的有害影响,也不影响体重。在6周的时间内,注射了载体或Ro-31-8220的野生型小鼠在分数缩短或任何其他心室尺寸测量方面没有变化(图5A和表1)。相比之下,与载体治疗或治疗前的基线值相比,6周注射Ro-31-8220的MLP - / -小鼠在分数缩短方面表现出显著的恢复(图5A,表1)。图5A所示的研究是在6个月大的野生型和MLP - / -小鼠中进行的。尽管在4周的治疗中,老龄MLP−/−小鼠(14个月)与给药小鼠相比,在部分缩短方面也有类似的改善(图5B,表1)。野生型和MLP−/−小鼠也接受了分离的、工作的心脏准备,显示长期给药Ro-31-8220的MLP−/−小鼠的心脏收缩力显著增加。与野生型小鼠相当(表2)。在舒张功能(- dP/dt)和左心室压力方面也观察到类似的恢复(表2)。 虽然Ro-31-8220在4周或6周的慢性给药期间挽救了MLP - / -小鼠的心脏收缩性能,但它没有改变心脏重量,也没有通过超声心动图评估逆转心室扩张,也没有改善组织病理学(数据未显示)。这些观察结果表明,与慢性Ro-31-8220治疗相关的心脏功能的部分增加可能涉及对收缩力本身的急性影响。事实上,在MLP−/−小鼠中注射Ro-31-8220仅3天,与载药治疗相比,分数缩短得到改善(图5C)。 |
||
| 酶活实验 |
蛋白激酶C(PKC)同工酶家族被认为在许多不同的细胞类型中介导了广泛的信号转导途径。一系列双吲哚基马来酰亚胺已被评估为传统PKC家族(PKCsα、β、γ)成员的抑制剂,以及新的Ca(2+)非依赖性PKC家族PKCε的代表性抑制剂。与吲哚咔唑星孢菌素相比,所有研究的双吲哚基马来酰亚胺对PKCα的选择性都比其他检测的同工酶低。此外,带有构象限制侧链的双吲哚基马来酰亚胺作为PKCε抑制剂的活性较低。其中最引人注目的是Ro 32-0432,它对PKCα的选择性是PKCε的10倍,对PKCβI的选择性是PKC-ε的4倍[2]。
|
||
| 细胞实验 |
对硫磷的活性代谢产物对氧磷是一种乙酰胆碱酯酶(AChE)抑制剂,通过凋亡机制杀死培养的小脑颗粒细胞神经元。蛋白激酶C是一种具有多种功能的酶,但其在对氧磷诱导的细胞死亡中的作用尚不清楚。我们发现,神经毒性浓度的对氧磷会增加PKC磷酸化。我们使用PKC激活剂佛波醇12-肉豆蔻酸13-乙酸酯(TPA)测试了PKC是否参与对氧磷诱导的神经元细胞死亡。TPA可增加PKC活性,并将对氧磷的神经毒性作用增强28%。与此形成鲜明对比的是,添加PKC抑制剂Ro-31-8220可以保护30%以上的神经元,否则这些神经元在治疗前或治疗后都会死于对氧磷诱导的神经元细胞死亡,并显著降低磷酸化PKC水平。研究还表明,Ro-31-8220的预处理完全阻断了对氧磷诱导的半胱氨酸天冬氨酸蛋白酶-3活性。这些结果表明,对氧磷神经毒性需要激活蛋白激酶C。[3]
在体外(DIV)8的指定时间内,将神经毒性浓度的对氧磷(200μM)添加到颗粒细胞培养物中。在DIV 8暴露于对氧磷之前或之后,将以下药物添加到颗粒细胞培养物中:在添加对氧磷前15分钟或后3小时添加Ro-81-3220(1μM)。在加入对氧磷前15分钟加入TPA(0.1μM)[1]。 |
||
| 动物实验 |
|
||
| 参考文献 |
|
||
| 其他信息 |
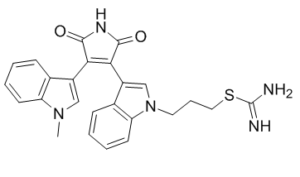
Ro 31-8220 is an imidothiocarbamic ester, a member of indoles and a member of maleimides. It has a role as an EC 2.7.11.13 (protein kinase C) inhibitor. It is functionally related to a maleimide.
Ro 31-8220 is a methanesulfonate salt form, a staurosporine analog that inhibits protein kinase C and induces apoptosis. The protein kinase C (PKC) family of isoenzymes is believed to mediate a wide range of signal-transduction pathways in many different cell types. A series of bisindolylmaleimides have been evaluated as inhibitors of members of the conventional PKC family (PKCs-alpha, -beta, -gamma) and of a representative of the new, Ca(2+)-independent, PKC family, PKC-epsilon. In contrast with the indolocarbazole staurosporine, all the bisindolylmaleimides investigated showed slight selectivity for PKC-alpha over the other isoenzymes examined. In addition, bisindolylmaleimides bearing a conformationally restricted side-chain were less active as inhibitors of PKC-epsilon. Most noticeable of these was Ro 32-0432, which showed a 10-fold selectivity for PKC-alpha and a 4-fold selectivity for PKC-beta I over PKC-epsilon.[1] The specificities of 28 commercially available compounds reported to be relatively selective inhibitors of particular serine/threonine-specific protein kinases have been examined against a large panel of protein kinases. The compounds KT 5720, Rottlerin and quercetin were found to inhibit many protein kinases, sometimes much more potently than their presumed targets, and conclusions drawn from their use in cell-based experiments are likely to be erroneous. Ro 318220 and related bisindoylmaleimides, as well as H89, HA1077 and Y 27632, were more selective inhibitors, but still inhibited two or more protein kinases with similar potency. LY 294002 was found to inhibit casein kinase-2 with similar potency to phosphoinositide (phosphatidylinositol) 3-kinase. The compounds with the most impressive selectivity profiles were KN62, PD 98059, U0126, PD 184352, rapamycin, wortmannin, SB 203580 and SB 202190. U0126 and PD 184352, like PD 98059, were found to block the mitogen-activated protein kinase (MAPK) cascade in cell-based assays by preventing the activation of MAPK kinase (MKK1), and not by inhibiting MKK1 activity directly. Apart from rapamycin and PD 184352, even the most selective inhibitors affected at least one additional protein kinase. Our results demonstrate that the specificities of protein kinase inhibitors cannot be assessed simply by studying their effect on kinases that are closely related in primary structure. We propose guidelines for the use of protein kinase inhibitors in cell-based assays.[2] Paraoxon, the active metabolite of parathion, is an acetylcholinesterases (AChE) inhibitor that kills cultured cerebellar granule cell neurons via an apoptotic mechanism. Protein kinase C is an enzyme with diverse functions but its role in paraoxon-induced cell death is unknown. We show that a neurotoxic concentration of paraoxon increases PKC phosphorylation. We tested whether PKC is involved in paraoxon-induced neuronal cell death by using the PKC activator, phorbol 12-myristate 13-acetate (TPA). TPA increases PKC activity and enhances the neurotoxic effect of paraoxon by 28%. In sharp contrast, addition of the PKC inhibitor Ro-31-8220 protects more than 30% neurons that would otherwise die from paraoxon-induced neuronal cell death in either a pretreatment or post-treatment paradigm and markedly reduces phospho-PKC pan levels. We also show that the pretreatment of Ro-31-8220 blocks paraoxon-induced caspase-3 activity completely. These results suggest that activation of protein kinase C is required for paraoxon neurotoxicity. [3] Background: The conventional protein kinase C (PKC) isoform alpha functions as a proximal regulator of Ca2+ handling in cardiac myocytes. Deletion of PKCalpha in the mouse results in augmented sarcoplasmic reticulum Ca2+ loading, enhanced Ca2+ transients, and augmented contractility, whereas overexpression of PKCalpha in the heart blunts contractility. Mechanistically, PKCalpha directly regulates Ca2+ handling by altering the phosphorylation status of inhibitor-1, which in turn suppresses protein phosphatase-1 activity, thus modulating phospholamban activity and secondarily, the sarcoplasmic reticulum Ca2+ ATPase. Methods and results: In the present study, we show that short-term inhibition of the conventional PKC isoforms with Ro-32-0432 or Ro-31-8220 significantly augmented cardiac contractility in vivo or in an isolated work-performing heart preparation in wild-type mice but not in PKCalpha-deficient mice. Ro-32-0432 also increased cardiac contractility in 2 different models of heart failure in vivo. Short-term or long-term treatment with Ro-31-8220 in a mouse model of heart failure due to deletion of the muscle lim protein gene significantly augmented cardiac contractility and restored pump function. Moreover, adenovirus-mediated gene therapy with a dominant-negative PKCalpha cDNA rescued heart failure in a rat model of postinfarction cardiomyopathy. PKCalpha was also determined to be the dominant conventional PKC isoform expressed in the adult human heart, providing potential relevance of these findings to human pathophysiology. Conclusions: Pharmacological inhibition of PKCalpha, or the conventional isoforms in general, may serve as a novel therapeutic strategy for enhancing cardiac contractility in certain stages of heart failure. [4] |
| 分子式 |
C25H23N5O2S
|
|
|---|---|---|
| 分子量 |
457.55
|
|
| 精确质量 |
457.157
|
|
| CAS号 |
125314-64-9
|
|
| 相关CAS号 |
Ro 31-8220 mesylate;138489-18-6
|
|
| PubChem CID |
5083
|
|
| 外观&性状 |
Typically exists as
Pink to red solid at room temperature
|
|
| 密度 |
1.42g/cm3
|
|
| LogP |
5.43
|
|
| tPSA |
193.95
|
|
| 氢键供体(HBD)数目 |
3
|
|
| 氢键受体(HBA)数目 |
4
|
|
| 可旋转键数目(RBC) |
7
|
|
| 重原子数目 |
33
|
|
| 分子复杂度/Complexity |
845
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
S(/C(=N/[H])/N([H])[H])C([H])([H])C([H])([H])C([H])([H])N1C([H])=C(C2C(N([H])C(C=2C2=C([H])N(C([H])([H])[H])C3=C([H])C([H])=C([H])C([H])=C23)=O)=O)C2=C([H])C([H])=C([H])C([H])=C12
|
|
| InChi Key |
DSXXEELGXBCYNQ-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C25H23N5O2S/c1-29-13-17(15-7-2-4-9-19(15)29)21-22(24(32)28-23(21)31)18-14-30(11-6-12-33-25(26)27)20-10-5-3-8-16(18)20/h2-5,7-10,13-14H,6,11-12H2,1H3,(H3,26,27)(H,28,31,32)
|
|
| 化学名 |
3-[3-[4-(1-methylindol-3-yl)-2,5-dioxopyrrol-3-yl]indol-1-yl]propyl carbamimidothioate
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|---|
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.1856 mL | 10.9278 mL | 21.8555 mL | |
| 5 mM | 0.4371 mL | 2.1856 mL | 4.3711 mL | |
| 10 mM | 0.2186 mL | 1.0928 mL | 2.1856 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
|
|---|
|