| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 500mg |
|
||
| 1g |
|
||
| 5g |
|
||
| 10g |
|
||
| 25g |
|
||
| Other Sizes |
|
| 靶点 |
Mitochondrial electron transport chain complex I
|
|---|---|
| 体外研究 (In Vitro) |
鱼藤酮影响 ENS 信号传导的方式涉及丝裂原激活多肽 (MAPK)、Toll 样受体、Wnt 和 Ras 的密切参与 [2]。
鱼藤酮抑制线粒体呼吸链复合体I可诱导多种细胞死亡。然而,其机制仍然难以捉摸。由于活性氧(ROS)在细胞凋亡中起重要作用,并且鱼藤酮抑制线粒体呼吸链复合体I被认为能够提高线粒体ROS的产生,我们研究了鱼藤酮诱导的细胞凋亡与线粒体活性氧之间的关系。鱼藤酮能够在HL-60细胞的分离线粒体和培养细胞中诱导线粒体复合体I底物支持的线粒体ROS产生。通过DNA断裂、细胞色素c释放和caspase 3活性证实鱼藤酮诱导的细胞凋亡。鱼藤酮诱导的细胞凋亡与鱼藤酮诱导的线粒体ROS产生之间存在定量相关性。抗氧化剂(谷胱甘肽、n -乙酰半胱氨酸和维生素C)可抑制鱼藤酮诱导的细胞凋亡。过量表达镁超氧化物歧化酶的HT1080细胞对鱼藤酮诱导的细胞凋亡的抗性比对照细胞更强,这也证实了鱼藤酮诱导的线粒体ROS在细胞凋亡中的作用。这些结果表明鱼藤酮能够通过增加线粒体活性氧的产生量来诱导细胞凋亡。[5] |
| 体内研究 (In Vivo) |
鱼藤酮可用于动物模型,以开发帕金森氏症的调节模型。鱼藤酮会导致兴奋性神经递质显着增加;在 PD 动物的小脑中检测到谷氨酸和天冬氨酸以及兴奋性 GABA、甘氨酸和牛磺酸显着减少 [1]。鱼藤酮(1.5、2 或 2.5 mg/kg)导致黑质中 α-突触核蛋白剂量需求增加。此外,在剂量为 2 和 2.5 mg/kg 时,鱼藤酮可诱导黑质中酪氨酸激酶免疫反应性神经元数量和纹状体中多巴胺数量显着减少 [4]。
|
| 酶活实验 |
呼吸测量[5]
如前所述,用克拉克氧电极测量耗氧量。简单地说,将1 × 107个HL-60细胞用不同浓度鱼藤酮处理30分钟。然后收集细胞并在含有0.3 m甘露醇、10 mm HEPES钾(pH 7.4)、5 mm磷酸钾(pH 7.4)和1 mm MgCl2的培养基中重悬。这些细胞被注射到一个呼吸室,然后被密封。呼吸室的总容积为1.6 ml。假设初始氧浓度为6.8 mg/l,测量并计算呼吸作用为氧浓度的变化率。细胞呼吸转化为对照的百分比。 ATP测定[5] 对于ATP的测量,使用市售的荧光素-荧光素酶测定试剂盒。简单地说,将HL-60细胞用不同浓度的鱼藤酮处理24 h,然后将其收集在1 ml的Eppendorf管中。用冰冷的PBS单次洗涤后,用试剂盒提供的体细胞atp释放试剂裂解细胞。加入荧光素底物和荧光素酶,在Perkin Elmer 3B荧光仪上评估生物发光。内标法测定全细胞ATP含量。细胞ATP水平转化为未处理细胞(对照)的百分比。 流式细胞术测定细胞超氧化物生成[5] 细胞超氧化物生成的测量如前所述,进行了一些修改。HL-60细胞用不同浓度的鱼藤酮处理30 min,收集细胞,250 × g汉克平衡盐溶液(Hank’s balanced salt solution, HBSS)洗涤,将细胞重悬于含有10 μm氢乙啶(hydroethidine, HE)的HBSS中,37℃孵养10 min。HT1080纤维瘤细胞用鱼藤酮处理30 min,然后取出培养液,HBSS洗涤一次。将细胞用含0.25%胰蛋白酶的HBSS孵育5分钟,再用含10 μm氢乙胺(HE)的HBSS重悬,37℃孵育10分钟。将所有细胞悬液置于12 × 75-mm管中检测。流式细胞术在Beckman-Coulter XL流式细胞仪上进行。采用610 nm长通滤光片采集乙锭荧光。 DNA碎片[5] DNA片段分析按照前面描述的方法进行,并进行了一些修改。在抗氧化剂存在或不存在的情况下,用不同浓度鱼藤酮处理HL-60细胞。细胞(2 × 107)用PBS(4°C, pH 7.4)洗涤一次,250 × g离心5分钟。然后用0.5 ml裂解缓冲液(10 mmTris-HCL, pH 7.4, 10 mm EDTA, 0.5%十二烷基硫酸钠)在冰上处理10分钟。37℃条件下,RNase A(终浓度100 μg/ml)作用1 h后,细胞在100 μg/ml蛋白酶k的存在下,50℃条件下孵育4 h,在溶液中加入50 μl 3 m醋酸钠(pH 5.2)和1 ml冷(4℃)100%乙醇沉淀DNA。然后收集DNA并溶解在TE缓冲液(10 mm Tris pH 8.0, EDTA 1 mm)中。在含有10 μg/ml溴化乙啶的1.2%琼脂糖凝胶上加载10 - 20 μl的DNA进行分析。电泳在0.5 × Tris硼酸EDTA缓冲液(18mm Tris碱基(pH 8.0), 18mm硼酸和1mm EDTA)中进行,在70 V下电泳2小时。在紫外光下观察DNA并拍照。 |
| 细胞实验 |
Cell Counting Kit-8 [2]
采用细胞计数试剂盒-8 (Cell Counting Kit-8, CCK8)检测鱼藤酮对ENS细胞活力的影响。96孔板每孔共接种2000个细胞,培养24 h,取出培养液,分别于浓度为0.01、0.02、0.07、0.21、0.62、1.85、5.56、16.67、50 μM的Rotenone/鱼藤酮环境下培养ENS细胞48 h,以未处理鱼藤酮的细胞为对照。然后,每孔加入10µL CCK-8,孵育3 h,用酶标仪检测450 nm处吸光度。 总RNA制备[2] 将0.3 μMRotenone/鱼藤酮处理/不处理48 h的细胞按TRIZOL法制备总RNA,用DNaseI去除总RNA中污染的DNA。采用NanoDrop 1000检测RNA浓度,每个样品取1 μg RNA,通过1%琼脂糖凝胶电泳分析RNA完整性,剩余RNA保存于- 80°C,用于RNA测序和实时聚合酶链反应(RT-PCR)。 |
| 动物实验 |
The aim of the present work was to investigate the neurochemical changes induced in the cerebellum of rat model of Parkinson's disease (PD). Rats were divided into two groups; control and rat model of PD induced by the intrastriatal injection of Rotenone. As compared to control, a significant increase in the excitatory amino acid neurotransmitters; glutamate and aspartate together with a significant decrease in the inhibitory amino acids, GABA, glycine and taurine were observed in the cerebellum of rat model of PD. This was associated with a significant increase in lipid peroxidation, nitric oxide and tumor necrosis factor-α and a significant decrease in reduced glutathione. A significant decrease in acetylcholinesterase and a significant increase in Na+,K+-ATPase were recorded in the cerebellum of rat model of PD. In addition the cerebellar sections from rat model of PD showed marked necrosis of Purkinje cells, irregular damaged cells, cytoplasmic shrinkage, necrosis and perineuronal vacuolation. The present results indicate that the disturbance in the balance between the excitatory and inhibitory amino acids may have a role in the pathogenesis of PD. According to the present neurochemical and histopathological changes, the cerebellum should be taken into consideration during the treatment of PD.[1]
Parkinson disease (PD) is a neurodegenerative disorder affecting mainly the motor system, as a result of death of dopaminergic neurons in the substantia nigra pars compacta. The present scenario of research in PD is directed to identify novel molecules that can be administered individually or co-administered with L-Dopa to prevent the L-Dopa-Induced Dyskinesia (LID) like states that arise during chronic L-Dopa administration. Hence, in this study, we investigated whether Morinda citrifolia has therapeutic effects in Rotenone-induced Parkinson's disease (PD) with special reference to mitochondrial dysfunction mediated intrinsic apoptosis. Methods: Male Sprague-Dawley rats were stereotaxically infused with Rotenone(3 µg in both SNPc and VTA) and co-treated with the ethyl acetate extract of Morinda citrifolia and levodopa.[3] Subcutaneous administration of Rotenone has recently attracted attention because of its convenience, simplicity and efficacy in replicating features of Parkinson's disease (PD) in animal models. However, the wide range of doses reported in the literature makes it difficult to evaluate the effectiveness of this technique objectively. The aim of the present study was to identify the optimum dose of subcutaneous Rotenone for establishing a model of PD. We injected male Wistar rats subcutaneously with one of three doses of Rotenone (1.5, 2, or 2.5mg/kg) daily for 5 weeks. Rotenone caused a dose-dependent increase in α-synuclein in the substantia nigra. Furthermore, at 2 and 2.5mg/kg, Rotenone caused a significant decrease in the number of tyrosine hydroxylase-immunoreactive neurons in the substantia nigra, and dopamine in the striatum. However, mortality at 2.5mg/kg was 46.7%, compared with just 6.7% at 2mg/kg; the high mortality observed at 2.5mg/kg would limit its application. The 2mg/kg dose showed no detrimental effect on body weight after 5 weeks of daily injections. Furthermore, rats in the 2mg/kg group showed a longer latency to descend from a horizontal bar and a grid wall, decreased rearing, and shorter latency to fall from a rotarod than rats that received vehicle or saline. Mitochondrial damage, observed by transmission electron microscopy, was also evident at this dose. Together, our data indicate that daily subcutaneous injection of 2mg/kg Rotenone in rats facilitates the formation of α-synuclein and reproduces the typical features of PD, while maintaining low mortality.[4] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Gastrointestinal absorption is low and incomplete. In animals, rotenone is hundreds of times more toxic intravenously than orally. Fats and oils increase absorption. Male and female Sprague Dawley rats were dosed with purified rotenone (99.23% pure). ... Preliminary excretion balance, excretion balance, pharmacokinetic, and enterohepatic circulation studies were conducted in both sexes using oral and intravenous (iv) doses of (14C)-rotenone at 0.01 to 5.0 mg/kg. Urinary and fecal metabolites were analyzed by thin layer chromatography. ... Feces were the major route of excretion for iv and oral exposures with small amounts of (14C) being recovered in the urine. Excretion was nearly complete in 48 hours in both sexes after iv doses and in males after oral doses. Female excretion was nearly complete 72 hours after oral dosing. Tissue retention of (14C) was low. The pharmacokinetics data were described by a two-compartment model. Data were consistent with enterohepatic recycling. ... Early experiments with rabbits and dogs fed with rotenone indicated retention and/or metabolism of the toxicant. No intact material was obtained from the urine, but the feces contained rotenone for at least 8 days after administration. A somewhat different picture emerged for mice 48 hr after treatment with (14)C-labeled rotenone, 20% of the (14)C was in the urine, 0.3% was expired, 5% remained in the body, and the rest was in the feces. When the fate and distribution of (14)C-labeled rotenone in different organs were followed in mice, 21.6% of the radioactivity was found in the small intestine, 19.5% in the urine, and 4.4% in the liver. Exhalation of (14)CO2 within 50 hr after oral or ip dosing of 5'beta-[3-methoxy-(14)C]rotenone to mice and rats respectively was 27 and 12.5%. /5'beta-[3-methoxy-(14)C]Rotenone/ Metabolism / Metabolites Male and female Sprague Dawley rats were dosed with purified rotenone (99.23% pure). ... Nine metabolites were detected in urine and feces. Four of these cochromatographed with (14)C-labeled impurities in the standard. No parent compound could be detected in feces or urine. The major metabolite (S0) was very polar and in feces accounted for 40.82 to 72.99% of the excreted (14)C by males and 33.48% to 65.76% by females. A similar polar metabolite in male urine accounted for 69.67% to 93.37% of the excreted (14)C and in females 43.51 to 94.88%. Only one metabolite (S8) from one rat comigrated with a known standard (rotenolone). It represented about 0.23% of the dose (8.2% of the urinary (14)C X 2.79% of the dose that was excreted in the urine). The principal metabolites formed both in vivo and in vitro from rotenone for these species are basically the same and are, in decreasing order of mammalian toxicity, as follows: 8'-hydroxyrotenone, 6a beta,12a beta-rotenolone, 6a beta,12a alpha-rotenolone, and 6',7'-dihydro-6',7'-dihydroxyrotenone. The hydroxylated metabolites also have reduced inhibitory activity to insect and rat liver mitochondria. The formation of water-soluble conjugates, which were more abundant in mammalian than in insect tissues, was also noted in these studies. A phenolic metabolite resulting from 3-O-demethylation was also identified. In vitro, rotenone is subject to a variety of cytochrome P-450-catalyzed oxidations in vertebrate and insect tissues. Oxidation occurs (e.g., at both the methyl group and the double bond of the isopropenyl group, and at the carbon atom adjacent to the keto group between the B and C rings to yield a pair of enantiomers, rotenolones I and II). Rotenone can also be oxidized completely to carbon dioxide (CO2) such that up to 13% and 27% of the compound administered to mice and rats appeared as exhaled. Some 20% of the ingested rotenone can be accounted for as urinary metabolites in rats and mice. For more Metabolism/Metabolites (Complete) data for ROTENONE (9 total), please visit the HSDB record page. Biological Half-Life ... The half-life of rotenone in the head, viscera, and carcass of bluegills was about 22, 11, and 28 days, respectively, and the major metabolites identified were rotenolone and 6',7'-dihydro-6',7'-dihydroxyrotenone. |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
Rotenone works by interfering with the electron transport chain in mitochondria. Specifically, it inhibits the transfer of electrons from iron-sulfur centers in complex I to ubiquinone. This prevents NADH from being converted into usable cellular energy (ATP). (L1274) Non-Human Toxicity Values LD50 Rabbit oral 1500 mg/kg LD50 Mouse oral 350 mg/kg LD50 Rat oral 25 mg/kg /oil solution/ LD50 Rat oral 60 mg/kg For more Non-Human Toxicity Values (Complete) data for ROTENONE (9 total), please visit the HSDB record page. |
| 参考文献 |
|
| 其他信息 |
Therapeutic Uses
Vet: ectoparasiticide Rotenone is also used to treat scabies and head lice on humans as well as various ectoparasites on livestock and pet animals. /Former use/ MEDICATION (VET): In veterinary medicine, rotenone is used in the power form to control parasitic mites on chickens and other fowl, and for lice and ticks on dogs, cats, and horses. |
| 分子式 |
C23H22O6
|
|---|---|
| 分子量 |
394.42
|
| 精确质量 |
394.141
|
| 元素分析 |
C, 70.04; H, 5.62; O, 24.34
|
| CAS号 |
83-79-4
|
| 相关CAS号 |
83-79-4
|
| PubChem CID |
6758
|
| 外观&性状 |
White to light yellow solid powder
|
| 密度 |
1.3±0.1 g/cm3
|
| 沸点 |
559.8±50.0 °C at 760 mmHg
|
| 熔点 |
159-164 °C(lit.)
|
| 闪点 |
244.6±30.2 °C
|
| 蒸汽压 |
0.0±1.5 mmHg at 25°C
|
| 折射率 |
1.591
|
| LogP |
4.65
|
| tPSA |
63.22
|
| 氢键供体(HBD)数目 |
0
|
| 氢键受体(HBA)数目 |
6
|
| 可旋转键数目(RBC) |
3
|
| 重原子数目 |
29
|
| 分子复杂度/Complexity |
664
|
| 定义原子立体中心数目 |
3
|
| SMILES |
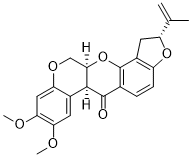
O=C1[C@H]2C3C=C(OC)C(OC)=CC=3OC[C@H]2OC2C3C[C@H](C(=C)C)OC=3C=CC1=2
|
| InChi Key |
JUVIOZPCNVVQFO-HBGVWJBISA-N
|
| InChi Code |
InChI=1S/C23H22O6/c1-11(2)16-8-14-15(28-16)6-5-12-22(24)21-13-7-18(25-3)19(26-4)9-17(13)27-10-20(21)29-23(12)14/h5-7,9,16,20-21H,1,8,10H2,2-4H3/t16-,20-,21+/m1/s1
|
| 化学名 |
(1S,6R,13S)-16,17-dimethoxy-6-prop-1-en-2-yl-2,7,20-trioxapentacyclo[11.8.0.03,11.04,8.014,19]henicosa-3(11),4(8),9,14,16,18-hexaen-12-one
|
| 别名 |
Ro-KO; Rotenone; Cube root; 83-79-4; Dactinol; Paraderil; Barbasco; Tubatoxin; (-)-Rotenone; Derris; Ronone; HSDB 1762; bCube-Pulver
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中(例如氮气保护),避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: ~50 mg/mL (~126.8 mM)
H2O: < 0.1 mg/mL |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (6.34 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: 2.5 mg/mL (6.34 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (6.34 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 2.5 mg/mL (6.34 mM) in 5% DMSO + 95% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.5354 mL | 12.6768 mL | 25.3537 mL | |
| 5 mM | 0.5071 mL | 2.5354 mL | 5.0707 mL | |
| 10 mM | 0.2535 mL | 1.2677 mL | 2.5354 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
|
|
|
|
|
|
|
|