| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|
| 靶点 |
Sarolaner (450 nM-1 mM; 5 minutes) suppresses currents produced by gamma-buffered butyric acid (GABA) on CfRDL-A285 and CfRDL-S285 receptors [1].
|
|---|---|
| 体外研究 (In Vitro) |
Sarolaner(450 nM-1 mM;5 分钟)抑制 CfRDL-A285 和 CfRDL-S285 受体上伽马缓冲丁酸 (GABA) 产生的电流 [1]。
在血液饲喂试验中,Sarolaner 对猫蚤 (C. felis felis) 表现出强效活性,LC80为0.3 µg/mL。[1] 在血液饲喂试验中,Sarolaner 对软蜱 (Ornithodoros turicata) 表现出强效活性,LC100为0.003 µg/mL。[1] 在与阿福拉纳(afoxolaner)和氟雷拉纳(fluralaner)的头对头比较体外试验中,sarolaner 对跳蚤(LC80 = 0.1 µg/mL)和软蜱(LC100 = 0.03 µg/mL)表现出更优的效力。[1] Sarolaner 能有效阻断稳定表达猫蚤RDL GABACl亚基的重组CHO-K1细胞中GABA诱导的电流。它没有表现出激动剂活性。其对敏感型CRDL-A285通道的抑制IC50为135 ± 33 nM,对耐药型CRDL-S285通道的抑制IC50为136 ± 16 nM。[1] 在相同试验的并排比较中,sarolaner (IC50 ~135-136 nM) 在抑制敏感和耐药跳蚤GABACl的GABA诱导电流方面,显著强效于阿福拉纳(afoxolaner)(IC50 ~412-539 nM)。[1] Sarolaner 对狄氏剂敏感和狄氏剂耐药(A285S突变)的跳蚤GABACl等位基因表现出相似的效力,这与狄氏剂不同,后者对耐药等位基因的效力显著降低。[1] |
| 体内研究 (In Vivo) |
Sarolaner 在 2.5 mg/kg 剂量下对 R 表现出 100% 的有效性;主侧壁。 sanguineus 以及 D. reticuLatus [1]。 Sarolaner 作用于 I,在 1.25-5 mg/kg(初级侧壁)时有效。蓖麻[1]。
在犬只中,单次口服2.5 mg/kg剂量(溶液)的sarolaner,在治疗后48小时对已有的猫蚤 (C. felis) 和血红扇头蜱 (Rhipicephalus sanguineus) 感染提供了100%的疗效。[1] 相同剂量(2.5 mg/kg)对每周再感染的猫蚤在35天内提供了≥99.9%的疗效。[1] 对于血红扇头蜱 (R. sanguineus),2.5 mg/kg剂量在35天内对每周再感染提供了100%的疗效。[1] 对于网纹革蜱 (Dermacentor reticulatus),2.5 mg/kg剂量在35天内对每周再感染提供了≥98.0%的疗效。[1] 在一项针对蓖子硬蜱 (Ixodes ricinus) 的剂量探索研究中,以1.25、2.5和5 mg/kg剂量口服混悬液给药的sarolaner,在治疗后48小时对已有感染提供了100%的疗效。[1] 针对随后的再感染,1.25 mg/kg剂量在35天内提供了≥99.3%的疗效(第57天疗效为80.1%)。2.5和5 mg/kg剂量在第57天前提供了>99.3%的疗效。[1] 在疗效研究中,未观察到犬只出现不良健康事件。[1] |
| 细胞实验 |
细胞活力测定[1]
细胞类型: CHO-K1 细胞 测试浓度: 450 nM-1 mM 孵育时间: 5分钟 实验结果:对敏感跳蚤(CfRDL-A285)和抗性跳蚤GABAC1引起的GABA电流的抑制,EC50分别为135和136 nM。 使用稳定表达重组猫蚤 (C. felis) RDL GABA门控氯通道亚基(CRDL-A285和CRDL-S285)的CHO-K1细胞进行电压钳电生理学研究。[1] 研究前立即制备细胞悬液。实验在自动化膜片钳平台上进行。外部溶液为HEPES缓冲盐溶液。内部溶液含有基于钾的盐和穿孔剂。所有记录在室温下进行,细胞保持-80 mV钳位电压。[1] 对于激动剂测试,将GABA在外部溶液中稀释。从储备液制备八点、3倍系列稀释液。通过集成移液器加入测试化合物。记录在加药前后采样。确定峰值内向电流。使用非线性回归拟合浓度反应数据以确定EC50。[1] 对于拮抗剂测试,构建测试化合物的八点浓度系列。加入测试化合物并记录后,加入约EC80浓度的GABA以评估测试化合物的抑制活性。记录在加药前后采样。计算GABA诱导电流的抑制百分比。构建浓度反应曲线并拟合以确定IC50。[1] |
| 动物实验 |
Animal/Disease Models: Dogs infected with R. sanguineus and D. reticulatus [1]
Doses: 2.5 mg/kg Route of Administration: po (oral gavage); 2.5 mg/kg One time Experimental Results:100% efficacy against R. sanguineus 48 hrs (hrs (hours)) after treatment , also has 98.0% efficacy against D. d. Net pattern. Animal/Disease Models: Ricin-infected dogs [1] Doses: 1.25, 2.5 and 5 mg/kg Route of Administration: po (oral gavage); 1.25, 2.5 and 5 mg/kg Primary Experimental Results: Prior to 7 days, all doses were effective in ricin Both narcotics demonstrated 100% efficacy and diminished subsequent reinfections by more than 99.3% by 57 days at doses of 5.0 and 2.5 mg/kg. An exploratory margin of safety study in adult Beagle dogs: Sarolaner was formulated in capsules with excipients (microcrystalline cellulose, sodium starch glycolate, sodium lauryl sulfate, magnesium stearate) and administered orally at doses of 2, 6, or 10 mg/kg. Each treatment group was dosed three times at 28-day intervals. A full post-mortem examination was conducted one week after the third dose. [1] An exploratory tolerance study in 8-week-old Beagle dogs: Sarolaner was formulated similarly in capsules and administered orally at doses of 4, 12, or 20 mg/kg. Each dog was dosed twice at a 28-day interval, followed by a complete post-mortem examination one week after the second dose. [1] A parallel-design bioavailability study in Beagle dogs: Sarolaner was administered either as an intravenous solution at 2 mg/kg or as a compressed tablet for oral administration (20 mg/dog, dose range 2.17–3.79 mg/kg). Dogs were fasted overnight prior to dosing. Blood samples for pharmacokinetic analysis were collected through Day 56. [1] Efficacy Study 1 in dogs: Dogs were treated via oral gavage with either a placebo control or a sarolaner solution (5 mg/mL in a tetraglycol/solutol base) to provide a dose of 2.5 mg/kg. [1] Efficacy Study 2 in dogs: Dogs were treated via oral gavage with either a placebo control, or a sarolaner suspension formulated in a carboxymethylcellulose/Tween-80 base with water at concentrations to provide doses of 1.25, 2.5, or 5 mg/kg. [1] |
| 药代性质 (ADME/PK) |
Following intravenous administration at 2 mg/kg to dogs, the volume of distribution at steady state (Vdss) was 2.81 L/kg and clearance (CL) was 0.12 mL/min/kg. [1]
Sarolaner was rapidly and well absorbed following oral dosing. Time to maximum plasma concentration (Tmax) occurred within the first day post-dose. [1] The oral bioavailability of sarolaner in fasted dogs was calculated to be >85%. [1] The elimination half-life (T1/2) of sarolaner was calculated to be 11–12 days. [1] Sarolaner exhibited dose-proportional plasma concentrations over the oral dose range of 1.25 to 5 mg/kg in dogs. [1] Sarolaner is highly protein bound (>99.9%). [1] Following oral administration of 20 mg/dog (dose-normalized to 2 mg/kg), the least squares mean maximum plasma concentration (Cmax) was 1100 ng/mL. [1] |
| 毒性/毒理 (Toxicokinetics/TK) |
In a mouse symptomatology screening model, sarolaner did not cause any adverse reactions at oral doses up to 30 mg/kg. [1]
In exploratory safety studies in dogs, oral administration of sarolaner at 2, 6, and 10 mg/kg three times at 28-day intervals to adult dogs, and at 4, 12, and 20 mg/kg twice at 28-day intervals to 8-week-old dogs, was well-tolerated at all dose levels with no adverse reactions observed. [1] Toxicokinetic evaluation indicated dose-proportional systemic exposure in both adult and young dogs. Test article-related changes in clinical pathology, gross pathology, organ weight, and histopathology were minimal and not considered adverse or clinically relevant. [1] |
| 参考文献 | |
| 其他信息 |
See also: Moxidectin; Pyrantel Pamoate; Sarolaner (component of); Sarolaner; Selamectin (component of).
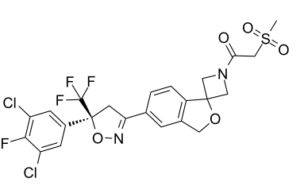
Drug Indication For the treatment of tick infestations (Dermacentor reticulatus, Ixodes hexagonus, Ixodes ricinus and Rhipicephalus sanguineus). The veterinary medicinal product has immediate and persistent tick killing activity for at least 5 weeks. For the treatment of flea infestations (Ctenocephalides felis and Ctenocephalides canis). The veterinary medicinal product has immediate and persistent flea killing activity against new infestations for at least 5 weeks. The veterinary medicinal product can be used as part of a treatment strategy for the control of Flea Allergy Dermatitis (FAD). For the treatment of sarcoptic mange (Sarcoptes scabiei). For the treatment of ear mite infestations (Otodectes cynotis). For the treatment of demodicosis (Demodex canis). Fleas and ticks must attach to the host and commence feeding in order to be exposed to the active substance. For the treatment of tick infestations (Dermacentor reticulatus, Ixodes hexagonus,Ixodes ricinus and Rhipicephalus sanguineus). The veterinary medicinal product has immediate and persistent tick killing activity for at least 5 weeks. , , For the treatment of flea infestations (Ctenocephalides felis and Ctenocephalides canis). The veterinary medicinal product has immediate and persistent flea killing activity against new infestations for at least 5 weeks. The veterinary medicinal product can be used as part of a treatment strategy for the control of Flea Allergy Dermatitis (FAD). , , For the treatment of sarcoptic mange (Sarcoptes scabiei). , , For the treatment of ear mite infestations (Otodectes cynotis). , , For the treatment of demodicosis (Demodex canis). , , Fleas and ticks must attach to the host and commence feeding in order to be exposed to the active substance. , Sarolaner is a novel isoxazoline ectoparasiticide specifically discovered and developed for use in companion animals (dogs). [1] The molecule is a chirally pure S-enantiomer. The flea and tick activity resides entirely in this enantiomer; the R-enantiomer is inactive. [1] The chemical structure consists of four subunits: a substituted phenyl head group, the isoxazoline core, a spiroazetidinebenzofuran moiety, and a methylsulfonylethanone tail. [1] It was discovered from a lead optimization program where over 3000 isoxazoline compounds were prepared and tested. [1] The primary mechanism of action is the inhibition of invertebrate ligand-gated chloride channels (GABA-gated chloride channels). [1] It is intended as a monthly oral treatment for the control of fleas and ticks on dogs. [1] |
| 分子式 |
C23H18CL2F4N2O5S
|
|---|---|
| 分子量 |
581.364037036896
|
| 精确质量 |
580.025
|
| CAS号 |
1398609-39-6
|
| PubChem CID |
73169092
|
| 外观&性状 |
White to off-white solid powder
|
| LogP |
4.781
|
| tPSA |
93.65
|
| 氢键供体(HBD)数目 |
0
|
| 氢键受体(HBA)数目 |
10
|
| 可旋转键数目(RBC) |
4
|
| 重原子数目 |
37
|
| 分子复杂度/Complexity |
1050
|
| 定义原子立体中心数目 |
1
|
| SMILES |
CS(=O)(=O)CC(=O)N1CC2(C1)C3=C(CO2)C=C(C=C3)C4=NO[C@@](C4)(C5=CC(=C(C(=C5)Cl)F)Cl)C(F)(F)F
|
| InChi Key |
FLEFKKUZMDEUIP-QFIPXVFZSA-N
|
| InChi Code |
InChI=1S/C23H18Cl2F4N2O5S/c1-37(33,34)9-19(32)31-10-21(11-31)15-3-2-12(4-13(15)8-35-21)18-7-22(36-30-18,23(27,28)29)14-5-16(24)20(26)17(25)6-14/h2-6H,7-11H2,1H3/t22-/m0/s1
|
| 化学名 |
(S)-1-(5'-(5-(3,5-dichloro-4-fluorophenyl)-5-(trifluoromethyl)-4,5-dihydroisoxazol-3-yl)-3'H-spiro[azetidine-3,1'-isobenzofuran]-1-yl)-2-(methylsulfonyl)ethan-1-one
|
| 别名 |
PF6450567; PF 6450567; PF-6450567
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~300 mg/mL (~516.03 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 7.5 mg/mL (12.90 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 75.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 7.5 mg/mL (12.90 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 75.0 mg/mL 澄清 DMSO 储备液加入900 μL 玉米油中,混合均匀。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.7201 mL | 8.6005 mL | 17.2010 mL | |
| 5 mM | 0.3440 mL | 1.7201 mL | 3.4402 mL | |
| 10 mM | 0.1720 mL | 0.8601 mL | 1.7201 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。