| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| Other Sizes |
|
| 靶点 |
ALK4 (IC50 = 1 μM); ALK5 (IC50 = 0.75 μM); ALK7 (IC50 = 2 μM)
|
|---|---|
| 体外研究 (In Vitro) |
SB-431542 可抑制 ALK4、ALK5 和 ALK7 活性,IC50 值分别为 1 μM、0.75 μM 和 2 μM [1]。 ALK5、淋巴结 I 型受体 ALK7 和激活素 I 型受体 ALK4(所有这些受体都与激酶结构域中的 ALK5 密切相关)均被 SB-431542 抑制(0–10 μM;24 小时)[1] 。 SB-431542(0.1、0.5、1、5 或 10 μM;30 分钟)可有效抑制 TGF-β 和激活素诱导的 Smad 磷酸化,但不能抑制 BMP4 [1]。 TGF-β 诱导的转录、基因表达、细胞凋亡和生长抑制均被 SB-431542 (0–10 μM) 抑制 [2]。
|
| 体内研究 (In Vivo) |
在新西兰兔中,SB-431542(结膜下;0.5 和 2 mM;第 1、2、3 和 7 天)可预防青光眼滤过手术后留下疤痕 [3]。
与对照组相比,实验组的IOP在手术后第25天之前一直保持较低水平(P<0.05)。组织学特征显示,实验组结膜下间隙只有轻微的胶原蛋白沉积。无论培养体系中是否存在TGF-β,SB-431542都能有效抑制细胞生长和迁移。SB-431542消除了TGF-β诱导的α-SM肌动蛋白、CTGF和Col I的上调。它有效地抑制了TGF-β刺激的Smad2的磷酸化,但不抑制MAPK通路成分的磷酸化。 结论:SB-431542抑制青光眼滤过术后瘢痕形成。其机制可能是SB-431542干扰Smad2的磷酸化,从而消除TGF-β诱导的成纤维细胞转分化,然后减少Col I的合成。[3] |
| 酶活实验 |
小分子抑制剂已被证明在研究信号转导途径方面非常有用,并有可能发展成为抑制信号转导途径的治疗方法,这些途径的活性会导致人类疾病。转化生长因子β(TGF-β)是多效性细胞因子大家族的成员,在正常和患病状态下参与许多生物过程,包括生长控制、分化、迁移、细胞存活、粘附和发育命运的指定。TGF-β超家族成员通过包含II型和I型受体(均为丝氨酸/苏氨酸激酶)的受体复合物发出信号。在这里,我们描述了一种小分子抑制剂(SB-431542),该抑制剂被鉴定为激活素受体样激酶(ALK)5(TGF-βI型受体)的抑制剂。我们证明它抑制ALK5以及激活素I型受体ALK4和淋巴结I型受体ALK7,这些受体在激酶结构域中与ALK5高度相关。它对识别骨形态发生蛋白(BMPs)的其他更不同的ALK家族成员没有影响。与此一致,我们证明SB-431542是内源性激活素和TGF-β信号传导的选择性抑制剂,但对BMP信号传导没有影响。为了证明SB-431542的特异性,我们测试了它对其他几种信号转导途径的影响,这些途径的活性取决于多种激酶的协同激活。SB-431542对ERK、JNK或p38 MAP激酶途径的成分或对血清激活的信号通路的成分没有影响[1]。
转录反应检测[2] 用CMV-βgal和p3TP-Lux或(CAGA)9MLP-Luc报告质粒瞬时转染FET细胞。将CMV-βgal、p21-Luc或PAI-1-Luc质粒瞬时转染HepG2细胞。在SB-431542存在下,将转染细胞在含有5 ng/ml TGF-β1的0.2%FBS中孵育22小时。细胞裂解物用于测量萤光素酶和β-gal活性,并给出了标准化的萤光素酶活性。 |
| 细胞实验 |
蛋白质印迹分析[1]
细胞类型: NIH 3T3 细胞; HaCaT、NIH 3T3、C2C12 细胞和 T47D 细胞 测试浓度: 10 μM; 0.1、0.5、1、5 或 10 μM 孵育时间: 24 小时; 30分钟 实验结果:有效抑制磷酸化Smad2。抑制 TGF-β 和激活素诱导的 Smad2 磷酸化,但不抑制 BMP 诱导的 Smad1 磷酸化。 细胞凋亡分析[2] 细胞类型: A549 和 HT29 细胞 测试浓度: 10 μM 孵育持续时间:24小时 实验结果:抑制TGF诱导的生长抑制和细胞凋亡。细胞侵袭分析[2] 细胞类型: A549 细胞 测试浓度: 2, 10 μM 孵育时间: 21 小时 实验结果: 阻断 TGF 诱导的肿瘤细胞侵袭。 细胞迁移测定 [2] 细胞类型: A549 细胞 测试浓度: 2, 10 μM 孵育时间:5小时、30小时 实验结果:阻断TGF诱导的肿瘤细胞迁移。 |
| 动物实验 |
Animal/Disease Models: Rabbits (3 to 5 months, 1.8 - 2.5 kg)[3]
Doses: 0.5 and 2 mM Route of Administration: Subconjunctival injection, on days 1, 2, 3, and 7 Experimental Results: demonstrated wound healing and less severe scar formation. To explore the inhibitive effect of SB-431542 (an ALK5 inhibitor) on scar formation after glaucoma surgery and to identify the potential pharmacologic target(s). Methods: Twenty-four New Zealand rabbits underwent filtration surgery on the right eye and were divided into a control group and three experimental groups (n=6). Human Tenon's fibroblast monolayer was scraped to generate a single gap, and then the control medium with SB-431542 only or containing 10 microg/L TGF-beta1 and SB-431542 (1-20 microM) was added. The cells were pretreated with SB-431542 or in control medium for 30 minutes before induction with 10 microg/L TGF-beta1 or 1 microg/L TGF-beta2. The expression of alpha-SM-actin, CTGF, and Col I, as well as changes in the Smad, ERK, P38, and AKT signaling pathways were detected.[3] |
| 参考文献 |
[1]. Gareth J Inman, et al. SB-431542 is a potent and specific inhibitor of transforming growth factor-beta superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol Pharmacol. 2002 Jul;62(1):65-74.
[2]. Sunil K Halder, et al. A specific inhibitor of TGF-beta receptor kinase, SB-431542, as a potent antitumor agent for human cancers. Neoplasia. 2005 May;7(5):509-21. [3]. Yi-qin Xiao, et al. SB-431542 inhibition of scar formation after filtration surgery and its potential mechanism. Invest Ophthalmol Vis Sci. 2009 Apr;50(4):1698-706. |
| 其他信息 |
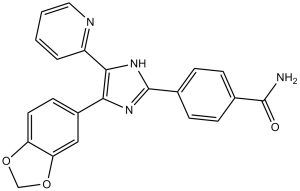
SB 431542 is a member of the class of benzamides that is 4-(imidazol-2-yl)benzamide carrying additional 1,3-benzodioxol-5-yl and pyridin-2-yl substituents at positions 4 and 5 respectively on the imidazole ring. It has a role as an EC 2.7.10.1 (receptor protein-tyrosine kinase) inhibitor. It is a member of benzamides, a member of imidazoles, a member of pyridines and a member of benzodioxoles.
Small molecule inhibitors have proven extremely useful for investigating signal transduction pathways and have the potential for development into therapeutics for inhibiting signal transduction pathways whose activities contribute to human diseases. Transforming growth factor beta (TGF-beta) is a member of a large family of pleiotropic cytokines that are involved in many biological processes, including growth control, differentiation, migration, cell survival, adhesion, and specification of developmental fate, in both normal and diseased states. TGF-beta superfamily members signal through a receptor complex comprising a type II and type I receptor, both serine/threonine kinases. Here, we characterize a small molecule inhibitor (SB-431542) that was identified as an inhibitor of activin receptor-like kinase (ALK)5 (the TGF-beta type I receptor). We demonstrate that it inhibits ALK5 and also the activin type I receptor ALK4 and the nodal type I receptor ALK7, which are very highly related to ALK5 in their kinase domains. It has no effect on the other, more divergent ALK family members that recognize bone morphogenetic proteins (BMPs). Consistent with this, we demonstrate that SB-431542 is a selective inhibitor of endogenous activin and TGF-beta signaling but has no effect on BMP signaling. To demonstrate the specificity of SB-431542, we tested its effect on several other signal transduction pathways whose activities depend on the concerted activation of multiple kinases. SB-431542 has no effect on components of the ERK, JNK, or p38 MAP kinase pathways or on components of the signaling pathways activated in response to serum.[1] Small molecule inhibitors of signaling pathways have proven to be extremely useful for the development of therapeutic strategies for human cancers. Blocking the tumor-promoting effects of transforming growth factor-beta (TGF-beta) in advanced stage carcinogenesis provides a potentially interesting drug target for therapeutic intervention. Although very few TGF-beta receptor kinase inhibitors (TRKI) are now emerging in preclinical studies, nothing is known about how these inhibitors might regulate the tumor-suppressive or tumor-promoting effects of TGF-beta, or when these inhibitors might be useful for treatment during cancer progression. We have investigated the potential of TRKI in new therapeutic approaches in preclinical models. Here, we demonstrate that the TRKI, SB-431542, inhibits TGF-beta-induced transcription, gene expression, apoptosis, and growth suppression. We have observed that SB-431542 attenuates the tumor-promoting effects of TGF-beta, including TGF-beta-induced EMT, cell motility, migration and invasion, and vascular endothelial growth factor secretion in human cancer cell lines. Interestingly, SB-431542 induces anchorage independent growth of cells that are growth-inhibited by TGF-beta, whereas it reduces colony formation by cells that are growth-promoted by TGF-beta. However, SB-431542 has no effect on a cell line that failed to respond to TGF-beta. This represents a novel potential application of these inhibitors as therapeutic agents for human cancers with the goal of blocking tumor invasion, angiogenesis, and metastasis, when tumors are refractory to TGF-beta-induced tumor-suppressor functions but responsive to tumor-promoting effects of TGF-beta.[2] Purpose: To explore the inhibitive effect of SB-431542 (an ALK5 inhibitor) on scar formation after glaucoma surgery and to identify the potential pharmacologic target(s). Methods: Twenty-four New Zealand rabbits underwent filtration surgery on the right eye and were divided into a control group and three experimental groups (n=6). Human Tenon's fibroblast monolayer was scraped to generate a single gap, and then the control medium with SB-431542 only or containing 10 microg/L TGF-beta1 and SB-431542 (1-20 microM) was added. The cells were pretreated with SB-431542 or in control medium for 30 minutes before induction with 10 microg/L TGF-beta1 or 1 microg/L TGF-beta2. The expression of alpha-SM-actin, CTGF, and Col I, as well as changes in the Smad, ERK, P38, and AKT signaling pathways were detected. Results: In comparison with the control rabbits, the IOPs in the experimental groups remained at lower levels until day 25 (P<0.05) after the surgery. Histologic profiles showed that there was only a mild deposition of collagen in the subconjunctival space in the experimental groups. The cell growth and migration were inhibited effectively by SB-431542, regardless of whether TGF-beta was present in the culture system. SB-431542 abrogated TGF-beta-induced upregulation of alpha-SM-actin, CTGF, and Col I. It effectively inhibited the phosphorylation of Smad2 stimulated by TGF-beta but not that of the components of the MAPK pathways. Conclusions: SB-431542 inhibits scar formation after glaucoma filtration surgery. The mechanism may be that SB-431542 interferes in the phosphorylation of Smad2, thus abrogating TGF-beta-induced fibroblast transdifferentiation and then decreasing Col I synthesis.[3] |
| 分子式 |
C22H16N4O3
|
|
|---|---|---|
| 分子量 |
384.39
|
|
| 精确质量 |
384.122
|
|
| 元素分析 |
C, 68.74; H, 4.20; N, 14.58; O, 12.49
|
|
| CAS号 |
301836-41-9
|
|
| 相关CAS号 |
SB-431542 (GMP);301836-41-9
|
|
| PubChem CID |
4521392
|
|
| 外观&性状 |
Off-white to yellow solid powder
|
|
| 密度 |
1.4±0.1 g/cm3
|
|
| 沸点 |
662.4±55.0 °C at 760 mmHg
|
|
| 熔点 |
214 °C(dec.)
|
|
| 闪点 |
354.4±31.5 °C
|
|
| 蒸汽压 |
0.0±2.0 mmHg at 25°C
|
|
| 折射率 |
1.680
|
|
| LogP |
4.28
|
|
| tPSA |
103.12
|
|
| 氢键供体(HBD)数目 |
2
|
|
| 氢键受体(HBA)数目 |
5
|
|
| 可旋转键数目(RBC) |
4
|
|
| 重原子数目 |
29
|
|
| 分子复杂度/Complexity |
582
|
|
| 定义原子立体中心数目 |
0
|
|
| InChi Key |
FHYUGAJXYORMHI-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C22H16N4O3/c23-21(27)13-4-6-14(7-5-13)22-25-19(20(26-22)16-3-1-2-10-24-16)15-8-9-17-18(11-15)29-12-28-17/h1-11H,12H2,(H2,23,27)(H,25,26)
|
|
| 化学名 |
4-[4-(1,3-benzodioxol-5-yl)-5-pyridin-2-yl-1H-imidazol-2-yl]benzamide
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (5.41 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (5.41 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: 2% DMSO+30% PEG 300+ddH2O: 5mg/mL 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.6015 mL | 13.0076 mL | 26.0152 mL | |
| 5 mM | 0.5203 mL | 2.6015 mL | 5.2030 mL | |
| 10 mM | 0.2602 mL | 1.3008 mL | 2.6015 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Cancer Res.2003 Nov 15;63(22):7791-8. |
|---|
Effects of SB-431542 and Gleevec on TGF-β-induced proliferation of NIH3T3 cells.Cancer Res.2003 Nov 15;63(22):7791-8. |
Effect of SB-431542 on TGF-β-stimulated responses in MG63 cells. Cancer Res.2003 Nov 15;63(22 |