| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| 5g |
|
||
| 10g | |||
| Other Sizes |

| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
AFTER ADMIN OF SKATOLE TO CATTLE IN A DOSE OF 0.1-0.2 G SKATOLE/KG INTRARUMINALLY OR 0.06 G/KG BY JUGULAR INFUSION, THE MEAN PLASMA CONCN OF SKATOLE BECAME MAXIMAL AT 3 AND 9 HR, RESPECTIVELY. GOATS WERE GIVEN A 2 HR JUGULAR INFUSION OF 3-METHYLINDOLE (3MI) CONTAINING (14)C-3MI USING PROPYLENE GLYCOL AS THE VEHICLE. 3MI WAS RAPIDLY CLEARED FROM BLOOD PLASMA AND TISSUES AFTER INFUSION, AND 81% OF THE RADIOACTIVITY WAS EXCRETED IN THE URINE BY 24 HR. MAX CONCN OF UNMETABOLIZED 3MI IN THE TISSUES RANGED FROM 2.6 TO 15 UG 3MI/G, INCL 7.5 UG 3MI/G IN THE LUNG. THE LUNG CONTAINED THE HIGHEST PROPORTION OF METABOLITES. THE DATA DEMONSTRATE THAT 3MI DOES NOT SELECTIVELY CONCENTRATE IN THE LUNG AND THAT THE CONCN ARE LOWER THAN THOSE USUALLY ASSOC WITH DIRECT MEMBRANE DAMAGE. Metabolism / Metabolites SKATOLE IS PRODUCED IN THE GI TRACT (SMALL INTESTINE AND RUMEN) BY THE BACTERIAL DEGRADATION OF DIETARY TRYPTOPHAN RESIDUES... MATURE BEEF COWS GRAZING ON DRY SUMMER RANGE WERE MOVED TO LUSH GREEN PASTURE TO INDUCE ACUTE BOVINE PULMONARY EDEMA AND EMPHYSEMA (ABPE) AND TO DETERMINE WHETHER PLASMA AND RUMINAL FLUID 3-METHYLINDOLE (3MI) CONCN ARE RELATED TO THE DEVELOPMENT OF ABPE. IN VITRO PRODN OF 3MI WAS OBSERVED IN CULTURE MEDIA INOCULATED WITH RUMINAL FLUID, DEMONSTRATING THAT MICROORGANISMS CAPABLE OF PRODUCING 3MI WERE IN THE RUMEN. APPARENTLY RUMINAL MICROORGANISMS OF CATTLE CONVERT TRYPTOPHAN (CONTAINED IN LUSH FORAGE) TO 3MI, WHICH UPON ABSORPTION BY THE ANIMAL MAY RESULT IN THE ONSET OF ABPE. FORMED FROM INDOLE-3-ACETIC ACID. YIELDS O-FORMAMIDOACETOPHENONE IN RAT, WHEAT; FRYDMAN RB ET AL; FEBS LETTERS 17: 273 (1971). YIELDS 5-HYDROXYSKATOLE, 7-HYDROXYSKATOLE IN RAT; DALGLIESH CE ET AL; BIOCHEM J 70: 13P (1958). YIELDS 6-HYDROXYSKATOLE IN RABBIT; JEPSON JB ET AL; BIOCHIM BIOPHYS ACTA 62: 91 (1962). YIELDS SALICYLIC ACID IN PSEUDOMONAS; PROCTOR MM; NATURE (LONDON) 181: 1345 (1958). /FROM TABLE/ GOATS WERE GIVEN JUGULAR INFUSIONS OF (14)C-3-METHYLINDOLE (3MI). A MAJOR ROUTE OF METABOLISM INVOLVED FORMATION OF 3-METHYLOXINDOLE AND SUGGESTS THAT A MIXED FUNCTION OXIDASE, PYRROLOOXYGENASE, MAY BE THE MAJOR METABOLIC SYSTEM INVOLVED. A MINOR ROUTE OF METABOLISM INVOLVED OXIDATION OF THE METHYL CARBON OF 3MI. For more Metabolism/Metabolites (Complete) data for 3-METHYLINDOLE (6 total), please visit the HSDB record page. 3-methylindole has known human metabolites that include 3-methylindole-2,3-epoxide and 3-methyleneindolenine. |
|---|---|
| 参考文献 | |
| 其他信息 |
Skatole is a methylindole carrying a methyl substituent at position 3. It is produced during the anoxic metabolism of L-tryptophan in the mammalian digestive tract. It has a role as a mammalian metabolite and a human metabolite.
3-Methylindole has been reported in Tachigali glauca, Tecoma stans, and other organisms with data available. See also: ... View More ... Mechanism of Action NUCLEOPHILIC THIOL AGENTS, GLUTATHIONE, L-CYSTEINE AND N-ACETYL-L-CYSTEINE, PROTECTED MICROSOMAL PROTEINS AGAINST ALKYLATION BY THE REACTIVE METABOLITE OF 3-METHYLINDOLE. THE CYTOSOL FRACTION FROM THE LUNGS OF CATTLE INCR THE PROTECTIVE EFFECT OF THESE THIOL AGENTS. PRETREATMENT OF SHEEP WITH DIETHYLMALEATE, WHICH DEPLETES GLUTATHIONE, INCR THE SEVERITY OF THE PNEUMOTOXIC EFFECT OF 3-METHYLINDOLE, WHEREAS PRETREATMENT WITH L-CYSTEINE DECR THE SEVERITY OF THIS EFFECT. THESE FINDINGS ARE CONSISTENT WITH A HYPOTHESIS THAT AN ELECTROPHILIC REACTIVE METABOLITE OF 3-METHYLINDOLE IS RESPONSIBLE FOR ITS PNEUMOTOXIC EFFECT AND IMPLIES THAT GLUTATHIONE AND GLUTATHIONE S-TRANSFERASES ARE INVOLVED IN THE DETOXIFICATION OF THIS REACTIVE METABOLITE. INCUBATION OF VARIOUS INDOLIC COMPD WITH GOAT LUNG MICROSOMES SHOWED THAT ONLY 3-METHYLINDOLE WAS ABLE TO GENERATE A FREE RADICAL IN THE NADPH-DEPENDENT MICROSOMAL SYSTEM, AS TESTED BY SPIN-TRAPPING. ENZYMIC RADICAL FORMATION FROM 3-METHYLINDOLE SUGGESTS A MICROSOMAL-ACTIVATED FREE RADICAL MECHANISM FOR THE SPECIFICITY OF 3-METHYLINDOLE-INDUCED PULMONARY TOXICITY. Bioactivation of 3-methylindole (3MI), a highly selective pneumotoxin in goats, was investigated in human lung and liver tissues in order to provide information about the susceptibility of humans to 3MI toxicity. Human lung microsomes were prepared from eight organ transplantation donors and liver microsomes from one of the donors were /selected/. The 3MI turnover rate with human lung microsomes was 0.23 +/- 0.06 nmol/mg/min, which was lower than the rate with the human liver microsomes (7.40 mnol/mg/min). The activities were NADPH dependent and inhibited by l-aminobenzotriazole, a potent cytochrome p450 suicide substrate inhibitor. Covalent binding of 3MI reactive intermediates to human tissues was determined by incubation of (14)C-3MI and NADPH with human lung and liver microsomal proteins. Although human lung microsomes displayed measurable covalent binding activity (2.74 +/- 2.57 pmol/mg/min), the magnitude of this reaction was only 4% as large as that seen with human liver microsomes and also was inhibited by l-aminobenzotriazole. Therefore, the bioactivation of 3MI to covalently binding intermediates is catalyzed by cytochrome p450 in human pulmonary tissues. These activities were compared to those activities measured with tissues from goats. Proteins from goat and human pulmonary and hepatic microsomal incubations were incubated with radioactive 3MI, and radioactive proteins were analyzed by SDS-PAGE and HPLC and visualized by autoradiography and radiochromatography, respectively. The results showed that a 57-kDa protein was clearly the most prominently alkylated target associated with 3MI reactive intermediates. These data suggest that humans may be susceptible to 3MI mediated toxicities and that the specificity of covalent binding and the extent of binding to target proteins may play important roles in organ and species selective susceptibilities to 3MI pneumotoxicity. Therapeutic Uses EXPTL USE: MEDICATION (VET): INJECTED IM FOR 15 CONSECUTIVE DAYS TO INFECTED GUINEA-PIGS WEIGHING 200-300 G, 5 MG SKATOLE/DAY EXHIBITED TUBERCULOSTATIC ACTIVITY AGAINST MYCOBACTERIUM TUBERCULOSIS HOMINIS. |
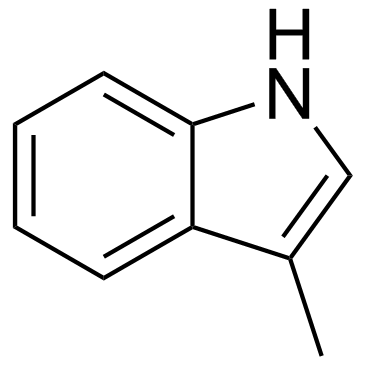
| 分子式 |
C9H9N
|
|---|---|
| 分子量 |
131.17446
|
| 精确质量 |
131.073
|
| CAS号 |
83-34-1
|
| 相关CAS号 |
Skatole-d3;111399-60-1;Skatole-d8;697807-03-7
|
| PubChem CID |
6736
|
| 外观&性状 |
Off-white to gray solid powder
|
| 密度 |
1.1±0.1 g/cm3
|
| 沸点 |
265.1±9.0 °C at 760 mmHg
|
| 熔点 |
92-97 °C(lit.)
|
| 闪点 |
112.5±11.3 °C
|
| 蒸汽压 |
0.0±0.5 mmHg at 25°C
|
| 折射率 |
1.655
|
| LogP |
2.6
|
| tPSA |
15.79
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
0
|
| 可旋转键数目(RBC) |
0
|
| 重原子数目 |
10
|
| 分子复杂度/Complexity |
122
|
| 定义原子立体中心数目 |
0
|
| InChi Key |
ZFRKQXVRDFCRJG-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C9H9N/c1-7-6-10-9-5-3-2-4-8(7)9/h2-6,10H,1H3
|
| 化学名 |
3-methyl-1H-indole
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~100 mg/mL (~762.37 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (19.06 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (19.06 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (19.06 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 7.6237 mL | 38.1185 mL | 76.2369 mL | |
| 5 mM | 1.5247 mL | 7.6237 mL | 15.2474 mL | |
| 10 mM | 0.7624 mL | 3.8118 mL | 7.6237 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。