| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| Other Sizes |
| 靶点 |
TLR7 (EC50 = 50 nM); human TLR7 (pEC50 = 7.3); Rat TLR7 (pEC50 = 6.6)
|
|---|---|
| 体外研究 (In Vitro) |
SM-324405 是一种新型治疗候选药物,旨在用于过敏免疫治疗[1]。
为了鉴定更有效的化合物,我们研究了在苯环上引入取代基。我们制备了以甲氧基为供电子基团、氟为吸电子基团的化合物。在对位(9i)引入甲氧基显著提高了EC50 8.1 nM的活性,而正异构体(9j)的活性大大降低(EC50 108 nM),但观察到代谢稳定性显著提高T1/2=98 min。如(9k)所示,引入氟原子使活性增加了2倍(EC50 25 nM)。T1/2增加到13.1 min。比较SM-324405/9e:H、9k:F和9i:OMe的血浆稳定性,观察到稳定性随着对位取代基OMe尺寸的增加而增加。>F>H.因此,我们推断酯周围的空间位阻对代谢率有重大影响,如之前对9g所示。我们将SM-324405/9e改为更大的乙酯9l,与9e相比,人血浆中的效力和不稳定性再次没有改善。因此,我们得出结论,甲酯在活性和代谢不稳定性之间达到了最佳平衡。 我们之前已经证明,替代的C(2)-取代基可以产生强效化合物,因此我们研究了在甲氧基乙氧基腺嘌呤类似物3上引入间苯乙酸。制备化合物17,并显示出与丁氧基类似物9e/SM-324405相当的不稳定性(T1/2 1.4分钟),同时保持效力。这些结果表明,N(9)位的间苯乙酸部分可能是各种类似物中有用的取代基,可以在保持TLR7活性的同时引入前药。 此外,还测量了对TLR8的体外活性。我们发现化合物9e的EC50超过10μM。除了人血浆中的特定效力和代谢不稳定外,我们还证实了SM-324405/9e在大鼠血浆中快速代谢(T1/2<0.04分钟)。这些数据促使我们选择化合物9e/SM-324405进行进一步评估。 通过以下方法测量化合物9e/SM-324405和相应酸16的体外IFN诱导活性。将人外周血单核细胞(PBMC)与化合物一起孵育,并通过人ELISA系统测量上清液中IFN的量。人PBMC中的IFN诱导活性如表3所示。[1] 化合物9e/SM-324405和16通过TLR7激活诱导IFN,化合物9e和相应的酸16在人PBMC中的最低有效浓度(MEC)分别为10和3000 nM。酯和酸的活性存在300倍的差异,因此我们认为9e显示了所需的前药特性[1]。 不同物种原代细胞中SM-324405和R848活性的比较[2] 我们进行了一项基因表达研究,以确定新的8-氧腺嘌呤化合物(如SM-324405)是否在多个物种的原代细胞中显示出活性。R848作为TLR7/8激动剂已被广泛研究(参考文献见引言),并被列为阳性对照。所有化合物都在被认为能产生最大反应的浓度下进行了测试。SM-324405在人PBMC以及大鼠和小鼠脾细胞中诱导了类似于R848诱导的基因诱导谱(图1)。TLR7刺激浆细胞样树突状细胞导致I型干扰素的产生,R848和SM-324405均诱导人外周血单个核细胞中IFNα和IFNβ的mRNA增加了10倍以上。小鼠和大鼠脾细胞对Ifnα和Ifnβ的诱导反应并不强烈,尽管在所有物种中,两种激动剂都明显诱导了Ifn调节基因Cxcl10、Ifit2和Oas。SM-324405和R848也在所有三个物种中诱导了一系列细胞因子和趋化因子基因,包括Ccl3、Il10、Il12、Ifnγ和TNF-α。Tlr7及其下游信号分子Myd88和Irf7在所有三种物种中也显示出两种激动剂的同等诱导水平。这些数据证实,从8-氧代腺嘌呤系列化合物中,SM-324405在人类、大鼠和小鼠细胞中的生物活性与R848相似。 小鼠mRNA数据未显示两种激动剂对Ifnα的影响。这可能是探针检测不良的结果,因此用R848和SM-324405和IFN-α刺激人PBMC和小鼠脾细胞,通过elisa或生物测定法测定(图2A和B)。数据证实,这两种激动剂都是IFN-α的诱导剂。此外,还测定了IFN-γ蛋白,结果表明,除了mRNA水平的变化外,蛋白质水平也有等效的影响(图2C和D)。在诱导人和小鼠细胞产生IFN-α和IFN-γ时,酸性代谢物的活性比母体化合物低至少10至30倍(图2)。 IFN-α的诱导与TLR7在浆细胞样树突状细胞上的激活是一致的。TLR7激动剂也被证明可以刺激B细胞的增殖,并评估了化合物对小鼠和大鼠脾细胞增殖的诱导作用(图3A和B)。R848和SM-324405显示出与SM-324405相似的活性,SM-324405在小鼠和大鼠中的pEC50值分别为8.4和8.2。在小鼠和大鼠脾细胞中,酸效力分别为6.8和<6.0,酯/酸效力比为40和>200。在用于确定细胞因子等产物的试剂有限的物种中,增殖反应的测定是活性的有用指标。因此,我们测定了狗PBMC中的活性(图3C),发现SM-324405的pEC50为7.9,表明这些化合物在狗体内具有活性。由于狗血浆缺乏能够切割SM-324405的酯酶,因此在狗PBMC中没有测定酸性代谢物的活性,因此这些化合物在该物种中不作为前药(数据未显示)。 AZ12441970改善了用药前的特性[2] 与母体(酯)相比,这些化合物中使用的前药概念依赖于活性降低的代谢物(酸),并且从人类和大鼠TLR7的报告分析(表1)中可以清楚地看出,SM-324405的酸充其量只比其母体酯低10倍。在确定该腺嘌呤系列化合物显示出与R848相当的生物活性后,我们寻找代谢物活性进一步降低的化合物,这导致了AZ12441970(表1)所示的一系列化合物。与SM-324405相比,该化合物的人TLR7效力降低了0.5个对数,但大鼠TLR7效力pEC50=6.6得以保持(表1)。酸性代谢产物AZ12443988的活性低于SM-324405的酸,对人和大鼠TLR7的酯/酸效力比均大于60倍。由于AZ12443988的活性极低,在高达10µM的浓度范围内无法确定pEC50,因此无法确定效力比值的真实范围。由于AZ12441970的血浆t1/2也比SM-324405短,因此总体而言,AZ124411970在体外表现出更好的前药特性。 AZ12441970对Th2细胞因子IL-5的抑制作用[2] TLR7激动剂具有通过重新平衡免疫反应来治疗以Th2表型为特征的过敏性疾病的潜力。我们使用PHA多克隆刺激人PBMC,并评估化合物抑制IL-5产生的能力,作为Th2细胞因子调节的标志物(图4A和B)。R848剂量依赖性地抑制IL-5的产生,pIC50为7.7。AZ12441970和SM-324405是IL-5的强效抑制剂,pIC50分别为8.7和7.9。与SM-324406(pIC50=6.8)相比,AZ12441970的酸性代谢产物(AZ12443988)作为IL-5产生抑制剂的活性要低得多(pIC50=5.4),AZ124419 70的酯/酸比为1900。这比SM-324405/SM-324406的13的比率有所改善。PHA在该试验中还诱导了IL-13,在评估10种TLR7激动剂时,我们观察到IL-5和IL-13的等效抑制作用(支持信息图S1)。然而,IL-4没有被诱导。在通过添加抗原(OVA)诱导IL-5的小鼠试验中,R848和AZ12441970有效地抑制了IL-5的产生,pIC50分别为8.7和7.5(图4C)。AZ12443988抑制IL-5的产生,pIC50为6.0。因此,这类TLR7激动剂抑制了人和小鼠T细胞中Th2细胞因子的产生。 |
| 体内研究 (In Vivo) |
SM-324405(9e,气管内给药)可有效防止全身细胞因子的释放,同时抑制过敏原诱导的气道炎症[1]。 SM-324405 的半衰期为 2.6 分钟,可在人血浆中转化为相应的酸 [1][2]。
为了鉴定更有效的化合物,我们研究了在苯环上引入取代基。我们制备了以甲氧基为供电子基团、氟为吸电子基团的化合物。在对位(9i)引入甲氧基显著提高了EC50 8.1 nM的活性,而正异构体(9j)的活性大大降低(EC50 108 nM),但观察到代谢稳定性显著提高T1/2=98 min。如(9k)所示,引入氟原子使活性增加了2倍(EC50 25 nM)。T1/2增加到13.1 min。比较SM-324405/9e:H、9k:F和9i:OMe的血浆稳定性,观察到稳定性随着对位取代基OMe尺寸的增加而增加。>F>H.因此,我们推断酯周围的空间位阻对代谢率有重大影响,如之前对9g所示。我们将SM-324405/9e改为更大的乙酯9l,与9e相比,人血浆中的效力和不稳定性再次没有改善。因此,我们得出结论,甲酯在活性和代谢不稳定性之间达到了最佳平衡。 我们之前已经证明,替代的C(2)-取代基可以产生强效化合物,因此我们研究了在甲氧基乙氧基腺嘌呤类似物3上引入间苯乙酸。制备化合物17,并显示出与丁氧基类似物9e/SM-324405相当的不稳定性(T1/2 1.4分钟),同时保持效力。这些结果表明,N(9)位的间苯乙酸部分可能是各种类似物中有用的取代基,可以在保持TLR7活性的同时引入前药。 此外,还测量了对TLR8的体外活性。我们发现化合物9e的EC50超过10μM。除了人血浆中的特定效力和代谢不稳定外,我们还证实了SM-324405/9e在大鼠血浆中快速代谢(T1/2<0.04分钟)。这些数据促使我们选择化合物9e/SM-324405进行进一步评估。 通过以下方法测量化合物9e/SM-324405和相应酸16的体外IFN诱导活性。将人外周血单核细胞(PBMC)与化合物一起孵育,并通过人ELISA系统测量上清液中IFN的量。人PBMC中的IFN诱导活性如表3所示。[1] 化合物9e/SM-324405和16通过TLR7激活诱导IFN,化合物9e和相应的酸16在人PBMC中的最低有效浓度(MEC)分别为10和3000 nM。酯和酸的活性存在300倍的差异,因此我们认为9e显示了所需的前药特性[1]。 |
| 酶活实验 |
TLR7报告基因检测[1]
HEK293-hTLR7细胞稳定转染人TLR7(pUNO表达载体)和pNiFty2 SEAP报告质粒,由阿斯利康公司赠送。将细胞以2×104个细胞/孔的速度接种在96孔板中,DMEM中补充了1%非必需氨基酸、10μg/mL杀芽素S、10μg/mL zeocin 和10%热灭活FCS,然后用不同浓度的测试化合物刺激,在37°C的5%CO2中孵育20小时。然后将磷酸对硝基苯酯作为底物加入板中,在室温下孵育20分钟。用1N氢氧化钠溶液停止反应后,用酶标仪测量405nm处的吸光度。 血浆稳定性研究[1] 将试验化合物加入到在37°C下预孵育5分钟的人或大鼠血浆中(化合物的最终浓度为1μM)。在37°C下孵育5或15分钟后,通过加入3倍体积的甲醇停止反应。然后将样品离心,通过LC-MS分析上清液中剩余的母体化合物。 人外周血单个核细胞(IFN诱导活性)[1] 肝素抗凝血液取自我们实验室的健康志愿者,他们在捐献前提供了知情同意书。按照制造商的建议,使用LymphoprepTM通过密度梯度离心分离PBMC。用PBS洗涤分离的PBMC两次,并用补充有50 U/mL青霉素/50μg/mL链霉素的无血清RPMI1640重新悬浮。将试验化合物溶解在DMSO中,并以不同浓度(最终DMSO浓度保持恒定在0.1%)加入PBMC的培养基中(1×106个细胞/mL)。在37°C、5%CO2下孵育18小时后,通过离心(1200 rpm,5分钟)收集上清液,并在-20°C下储存,直至分析细胞因子。用ELISA试剂盒测定IFN。 |
| 细胞实验 |
TLR报告分析[2]
用人TLR7(pUNO表达载体)和pNiFty2 SEAP报告质粒稳定转染的HEK293细胞保持在Dulbecco改良的Eagle培养基中,FCS 10%(v/v),2 mM l-谷氨酰胺,非必需氨基酸,10µg·mL-1杀稻瘟素s和10µg•mL-1 zeocin。使用的序列由欧洲分子生物学实验室核苷酸序列数据库序列AF240467表示。将细胞以每孔10000个细胞的速度接种在经组织培养处理的透明平底聚苯乙烯96孔板中。通过添加试验化合物并在37°C、5%CO2的环境中孵育20小时,生成了剂量-反应曲线。使用对硝基苯磷酸盐作为底物定量释放的分泌性碱性磷酸盐(SEAP),并通过微孔板读数器测定405nm处的吸光度。 血浆稳定性测定[2] 在37°C下将试验化合物(初始浓度为1µM)加入到人或大鼠血浆(通过离心1800×g EDTA管中收集的血液制备)中,总体积为0.5mL。在37°℃下孵育10分钟,在0、20秒、40秒和1、2、3、5和10分钟时取样,放入乙腈中。通过LC/MS/MS分析上清液中剩余的母体化合物,并确定母体化合物的t1/2。 脾细胞制剂[2] 在颈椎脱位后,从二氧化碳窒息的雄性棕色挪威大鼠或天真的雌性Balb/c小鼠(Harlan)身上取出脾脏,并将其放入含有RPMI 1640的培养皿中。将脾脏轻轻推入70µm BD Falcon细胞株中,以获得单细胞悬浮液。将细胞在400×g下离心5分钟以获得细胞沉淀,去除上清液并将细胞重新悬浮在新鲜的RPMI 1640中。再次离心细胞,将细胞重新悬浮在完全培养基(RPMI-1640、5%(v/v)胎牛血清(FCS)、2 mM l-谷氨酰胺、10 U·mL-1青霉素、10µg·mL-1链霉素和50µM 2-巯基乙醇)中。 外周血单个核细胞(PBMC)制剂[2] 将健康、自愿的志愿者的血液收集到肝素中,分层到淋巴细胞分离培养基1077(PAA,Pasching,Austria)上,以700×g离心25分钟。去除PBMC层,用PBS稀释至50 mL,以400×g离心10分钟。去除上清液,将沉淀重新悬浮在50 mL PBS中,以300×g离心5分钟。最后用50 mL PBS洗涤细胞,以200×g离心回收细胞5分钟。最终将PBMC重新悬浮在测定培养基(RPMI 1640,含25 mM HEPES,FCS 10%(v/v),2 mM l-谷氨酰胺,10 U·mL-1青霉素)中。10µg·mL-1链霉素)。 从收集到肝素中的狗血(动物设施,阿斯利康研发)制备狗PBMC,并使用与人PBMC相同的方案制备PBMC。 脾细胞培养[2] 将20微升试验化合物或含有1%(v/v)DMSO的完整RPMI 1640(载体对照)加入每个孔中,然后加入180µL脾细胞悬浮液(2×105个细胞),如前所述制备完整RPMI。脾细胞和化合物在37°C的空气/CO2(95/5 v/v)气氛中孵育一段时间。 在44小时时,通过在细胞测定中加入0.0185MBq[3H]-胸苷来测定脾细胞增殖。再孵育6小时后,使用Tomtec过滤装置将细胞收集到玻璃纤维过滤垫上。将垫子干燥,加入Betaplate Scint,用MicroBeta 1450 Trilux定量过滤器结合的放射性。 小鼠脾细胞IL-5和IFN-γ[2] 在第0天,通过腹腔注射10µg OVA+1 mg Al(OH)3的100µL溶液对幼年雌性Balb/c小鼠进行免疫接种。免疫接种8天后,将OVA/Al(OH)3致敏小鼠的脾脏收集到RPMI 1640培养基中,并如前所述制备和孵育脾细胞。加入OVA至终浓度为1mg OVA mL-1,孵育5天。去除上清液以测定产生的IL-5和IFN-γ的量。 PBMC培养[2] 向每个孔中加入20微升含有1%(v/v)二甲亚砜(DMSO)的试验化合物或试验培养基,作为载体对照,然后在试验培养基(200000个细胞)中加入180µL PBMC细胞悬浮液(如前所述制备)。PBMC和化合物在37°C的空气/CO2(95/5 v/v)气氛中孵育规定的时间。 为了诱导IL-5,制备人PBMC并用如前所述的化合物铺板。以5µg·mL-1的终浓度加入植物血凝素(PHA),并在去除上清液后孵育44小时,以测定产生的IL-5的量。 在添加丁酰胆碱酯酶(BChE)以缩短前药暴露时间的试验中,用浓度为1 U·mL-1的BChE接种PBMC,并通过添加化合物启动培养。24小时后,取出150µL上清液进行细胞因子测定,并用150µL新鲜培养基替换。在44小时时,加入[3H]-胸苷,并如前所述测定增殖。 基因芯片分析[2] Balb/c小鼠脾细胞、Brown Norway大鼠脾细胞或人PBMC与化合物一起孵育,4小时后用TRIzol®试剂提取RNA。根据标准方案,对人类(HG-U133+2)、小鼠(MOE430)和大鼠(RAE230)Affymetrix芯片组进行微阵列分析。在GeneChip Operating Software中使用MAS5算法对原始微阵列数据进行归一化。 |
| 动物实验 |
Inhibition of Inflammatory Cells in BALF (Efficacy) [1]
Male 8−10 weeks old Brown Norway rats were sensitized by intraperitoneal injection of ovalbumin (1 mg) together with aluminum hydroxide adjuvant (100 mg) in saline (1 mL) on day 0 and 7. Control (unsensitized/unchallenged) animals received vehicle (saline) alone at the same time points. On any one-day between days 14 and 18, rats were challenged by exposure to ovalbumin aerosol for 15 min generated from a 10 mg/mL ovalbumin solution by a nebulizer. Control animals were similarly exposed to saline aerosol for 15 min. Two hours before antigen challenge, rats were dosed with test compounds (suspended in saline) or vehicle by i.t. administration (dosing volume was 0.5 mL/kg). Twenty-four hours after antigen challenge, rats were sacrificed and the trachea was cannulated. The airway lumen was washed with 2 mL of saline, and this procedure was repeated six times (total volume of 12 mL). Infiltrated cells in BALF were stained with Turk solution and the number of nucleated cells was counted in a counting chamber. A differential count was made on a smear prepared with a cytocentrifuge and stained with Diff-Quick solution (May−Grunwald stain). At least 300 cells were counted in each BALF sample (magnification × 400). Induction of Systemic IFN (Side Effect) [1] Male 8−10 weeks old Brown Norway rats were dosed with test compounds (suspended in saline) by i.t. administration (dosing volume was 0.5 mL/kg). At 2, 4, 6, and 24 h after i.t. administration, rats were anaesthetized with ether, and heparinized blood samples (about 0.3 mL) were collected via the caudal vein. Then plasma samples were prepared by centrifugation (12000 rpm for 10 min), and stored at −20 °C until analyzed for IFN. IFN titers in the plasma samples were determined in a CPE reduction assay (bioassay) using L929 and vesicular stomatitis virus (VSV). Determination of pharmacokinetics In vivo [2] AZ12441970 was formulated in 0.1% Tween80/0.6% NaCl/50 mM phosphate buffer pH 6.0 at a concentration of 0.5 mg·mL−1. Six female BALB/c mice were briefly anaesthetized with isoflurane then dosed intranasally with 50 µL of the formulation, giving a dose of 1 mg·kg−1 per mouse. This volume is sufficient to be inhaled into the lung rather than remain in the nasal cavity. At each time point, two animals were killed by an overdose of pentobarbital and blood taken from the vena cava into sodium fluoride (0.2 M final concentration) to prevent hydrolysis by esterase enzymes before mixing with the anticoagulant EDTA. Samples were quenched in methanol and frozen at −20°C. Lungs were excised and placed in vials containing 1 mL sodium fluoride (1.2 M) and immediately frozen at −20°C. The lungs were homogenized with eight parts water, and aliquots of the homogenate quenched with methanol. Standard curves were prepared from a known weight of the test compound AZ12441970 and the acid metabolite AZ12443988, added to lung homogenate or blood containing sodium fluoride and treated as earlier samples. All samples were centrifuged and the supernatant analysed by LC/MSMS and the concentrations of AZ12441970 and AZ12443988 quantified. Mouse OVA-induced allergic airways model [2] Female C57BL/6 mice were sensitized by subcutaneous injection of 10 µg of OVA adsorbed with 4 mg aluminium hydroxide adjuvant in 100 µL on Day 0 and 14. Animals were challenged by intratracheal (20 µL) administration of OVA (0.5 mg·mL−1) on Day 22. AZ12441970 (40 µL, dissolved in 0.1% Tween80/ 0.6% NaCl/50 mM phosphate buffer pH 6.0) was administered via the intratracheal route 24 h and 2 h prior to OVA challenge. Animals were killed under anaesthesia 48 h after the OVA challenge, and the number of eosinophils in bronchoalveolar lavage fluid was measured by FACS analysis as described previously (van Rijt et al., 2004). Briefly, bronchoalveolar lavage fluid cells were pre-incubated with anti-mouse CD16/CD32 monoclonal antibody 2.4G2 (BD Bioscience, San Diego, CA, USA) at 4°C for 15 min, then incubated with anti-mouse FITC-CD4(L3T4), FITC-CD8, FITC-B220 and PE-CCR3 (BD Bioscience). Number of CD4− CD8− B220− CCR3+ eosinophils was determined using a Becton Dickinson FACScan (Becton Dickinson). IL-5 in bronchoalveolar lavage fluid was measured by elisa (BD Bioscience). Systemic cytokine induction in mice [2] AZ12441970 and R848 (dissolved in 0.1% Tween80/0.6% NaCl/50 mM phosphate buffer pH 6.0) were administered to naïve female C57BL/6 mice via the intratracheal route in a volume of 20 µL per mouse. Blood was collected 90 min later into heparinized syringes and plasma was prepared by centrifugation. Plasma was stored frozen until analysis. IFN-α in plasma was measured by the reporter assay system using L929/OAS cells and cytokines were determined by elisa or Luminex technologies. |
| 药代性质 (ADME/PK) |
Characterization of the TLR agonist activity of SM-324405, AZ12441970 and their metabolites [2]
A synthetic chemistry program was undertaken that led to TLR7 agonist antedrugs that were rapidly metabolized in plasma (Kurimoto et al., 2010). A subsequent research programme led to the identification of an alternative series of compounds exemplified by AZ12441970. This paper profiles the biological activity and mechanism of action of this series as demonstrated by the TLR7 agonists, SM-324405 and AZ12441970, along with their metabolites (Table 1). These compounds were rapidly metabolized in human plasma with a t1/2 of 1–3 min and in rat plasma with a t1/2 of less than 1 min (Table 1). AZ12441970 and SM-324405 showed activity that was equivalent to or greater than the well characterized TLR7/8 ligand, R848, in human and rat TLR7 reporter assays (Table 1). Whereas R848 was active at human TLR8, neither of the other two compounds had human TLR8 activity, as assessed by ability to activate NF-κB in a reporter cell line. The product generated following metabolism in plasma is an acid that, in the case of SM-324405, still showed significant activity in the human and rat TLR7 reporter assays. However, the design of AZ12441970 was such that its acid metabolite, AZ12443988, showed a greater than 60-fold reduction in potency in the human TLR7 reporter assay. Neither of the acids showed any detectable TLR8 activity. |
| 参考文献 |
|
| 其他信息 |
Systemic administration of a Toll-like receptor 7 (TLR7) agonist is effective to in suppressing Th2 derived inflammation, however systemic induction of various cytokines such as IL-6, IL-12, and type I interferon (IFN) is observed. This cytokine induction would be expected to cause flu-like symptoms. We have previously reported adenine compounds (3, 4) as interferon inducing agents acting as TLR7 agonists. To identify potent anti-inflammatory compounds without systemic side effects, a labile carboxylic ester as an antedrug functionality onto the N(9)-benzyl group of the adenine was introduced. We found that 9e was a potent TLR7 agonist (EC(50) 50 nM) and rapidly metabolized by human plasma (T(1/2) 2.6 min) to the pharmacologically much less active carboxylic acid 16. Intratracheal administration of 9e effectively inhibited allergen-induced airway inflammation without inducing cytokines systemically. Therefore, the TLR7 agonist with antedrug characteristics 9e (SM-324405) is a novel candidate for immunotherapy of allergic diseases. [1]
In this paper, the research for a novel class of agent for immunotherapy of allergic disease was described. A series of 2-butoxy-8-oxoadenines containing an ester moiety were prepared and evaluated for TLR7 agonist potency and stability in human plasma. We found that compound 9e/SM-324405 exhibited good potency and was rapidly metabolized to the much less active carboxylic acid 16. Intratracheal administration of 9e in a BN rat model significantly reduced the number of eosinophils in BALF and did not induce IFN systemically. [1] We have identified potent TLR7 agonists antedrugs and shown data which indicate that the TLR7 agonist 9e with antedrug properties is a novel candidate for the immunotherapy of allergic diseases. BACKGROUND AND PURPOSE Toll-like receptor 7 (TLR7) agonists have potential in the treatment of allergic diseases. However, the therapeutic utility of current low molecular weight TLR7 agonists is limited by their systemic activity, resulting in unwanted side effects. We have developed a series of TLR7-selective 'antedrugs', including SM-324405 and AZ12441970, which contain an ester group rapidly cleaved in plasma to reduce systemic exposure. EXPERIMENTAL APPROACH Agonist activity at TLR7 of the parent ester and acid metabolite was assessed in vitro in reporter cells and primary cells from a number of species. Pharmacokinetics following a dose to the lungs was assessed in mice and efficacy evaluated in vivo with a mouse allergic airway model. KEY RESULTS Compounds were selective agonists for TLR7 with no crossover to TLR8 and were metabolically unstable in plasma with the acid metabolite showing substantially reduced activity in a number of assays. The compounds inhibited IL-5 production and induced IFN-α, which mediated the inhibition of IL-5. When dosed into the lung the compounds were rapidly metabolized and short-term exposure of the 'antedrug' was sufficient to activate the IFN pathway. AZ12441970 showed efficacy in a mouse allergic airway model with minimal induction of systemic IFN-α, consistent with the low plasma levels of compound. CONCLUSIONS AND IMPLICATIONS The biological and metabolic profiles of these TLR7-selective agonist 'antedrug' compounds are consistent with a new class of compound that could be administered locally for the treatment of allergic diseases, while reducing the risk of systemic side effects. LINKED ARTICLE This article is commented on by Kaufman and Jacoby, pp. 569-572 of this issue. To view this commentary visit http://dx.doi.org/10.1111/j.1476-5381.2011.01758.x.[2] As another strategy, an antedrug is defined as a locally active compound that is designed to be rapidly metabolized to an inactive form upon entry into the circulation and prevents systemic toxicity by losing its agonistic activity in a plasmatic environment. A TLR agonistic antedrug has a cleavable linker at the critical TLR binding moiety. The cleavable linker is responsive to the plasmatic environment, so the TLR agonist is inactivated by loss of the critical binding moiety. SM-324405 and AZ12441970 are TLR 7 agonistic antedrugs that have ester bonds at their critical TLR binding moiety. The esters are easily cleaved by plasma esterase, resulting in reduced agonistic activity. The loss of the TLR binding moiety results in a decrease in induced interferon-α (IFN-α) secretion by peripheral blood mononuclear cells (PBMCs).[3] |
| 分子式 |
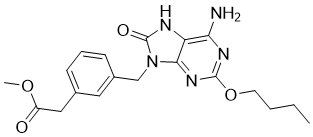
C19H23N5O4
|
|---|---|
| 分子量 |
385.42
|
| 精确质量 |
385.175
|
| 元素分析 |
C, 59.21; H, 6.02; N, 18.17; O, 16.60
|
| CAS号 |
677773-91-0
|
| PubChem CID |
23079483
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.4±0.1 g/cm3
|
| 沸点 |
654.0±65.0 °C at 760 mmHg
|
| 闪点 |
349.3±34.3 °C
|
| 蒸汽压 |
0.0±2.0 mmHg at 25°C
|
| 折射率 |
1.645
|
| LogP |
1.55
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
7
|
| 可旋转键数目(RBC) |
9
|
| 重原子数目 |
28
|
| 分子复杂度/Complexity |
548
|
| 定义原子立体中心数目 |
0
|
| SMILES |
CCCCOC1=NC(=C2C(=N1)N(C(=O)N2)CC3=CC=CC(=C3)CC(=O)OC)N
|
| 别名 |
SM-324405; SM 324405; 677773-91-0; SM 324,405; SM-324,405; Methyl3-[(6-amino-2-butoxy-7,8-dihydro-8-oxo-9H-purin-9-yl)methyl]benzeneacetate; CHEMBL1089224; Methyl 2-(3-((6-amino-2-butoxy-8-oxo-7,8-dihydro-9H-purin-9-yl)methyl)phenyl)acetate; Methyl 3-[(6-amino-2-butoxy-7,8-dihydro-8-oxo-9H-purin-9-yl)methyl]benzeneacetate; methyl 2-[3-[(6-amino-2-butoxy-8-oxo-7H-purin-9-yl)methyl]phenyl]acetate; SM324405
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~50 mg/mL (~129.73 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (6.49 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL 澄清 DMSO 储备液加入900 μL 玉米油中,混合均匀。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.5946 mL | 12.9729 mL | 25.9457 mL | |
| 5 mM | 0.5189 mL | 2.5946 mL | 5.1891 mL | |
| 10 mM | 0.2595 mL | 1.2973 mL | 2.5946 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
|
|