| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 100mg |
|
||
| Other Sizes |
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Rapidly absorbed, widely distributed and mostly excreted within 48 hr. Groups of Wistar Hsd/Cpb: Wu rats (about 200 g at treatment) were dosed with labeled spirodiclofen (radiopurity > 98%) in 10 mL/kg of 0.5% CMC suspension as follows (showing group designations in brackets): [6] single high dose (100 mg/kg, 4 M); [7] single low dose (including CO2 measurement) (2 mg/kg, 4 M); [8] single low dose (EPA basic test) (2 mg/kg, 4 M); [9] single low dose (2 mg/kg, 4 F); [10] 14 daily doses with 2 mg/kg/day non-radioactive a.i., then 1 labeled low dose (2 mg/kg, 4 M); and [13] single low dose, bile cannulation study (2 mg/kg, 6 M). In low dose groups, about 70% of administered dose was absorbed, with most of the label found in the urine, and about 12% of administered dose found in the bile. In the high dose (100 mg/kg) group, 61% of label was found in feces, vs. 35% in urine, suggesting saturable absorption at high dose levels. Very little label (0.05% of administered dose) was found in exhaled CO2. Peak plasma concentrations were observed between 2.5 hr to 3.9 hr in low dose groups, vs. 5.6 hr in high dose rats. This study employed 2 male monkeys, each dosed with about 0.2 mg/kg spirodiclofen in a single treatment. The iv treatment was prepared as a PEG 200 solution in water, and the dermal treatment was a suspension of fine spirodiclofen crystals in water. The patch for the dermal treatment was removed after 8 hr, after which the application area was washed with 1% Ivory detergent solution followed by tape stripping and alcohol swab wiping. Monkeys were maintained in metabolism cages after dosing (except that the first 8 hr after the iv treatment was spent in a primate chair). Following iv dosing, urinary excretion was rapid: 64% of administered dose was obtained in urine within the first 8 hr, with an additional 18% in the next 16 hr. A total of 87% of dose was obtained in urine, and an additional 15% in cage debris/rinse (attributed primarily to urine). About 5% of administered dose was found in feces in the iv test. Measured recovery was slightly more than theoretical. Following dermal treatment, 1.1% of administered dose was found in urine, 0.3% in cage wash, and 0.2% in feces. Most of the dermally administered dose was found in the detergent swab process. An additional 9% was found in the patch or containment dome, and 10% was obtained with the alcohol swab step. Thus the dermal response from this one subject suggested only about 1.6% total absorption. This study employed 5 male monkeys, each dosed dermally with an average of 0.04 mg/kg spirodiclofen as the SC 240 formulation in a single treatment, considered to represent a plausible field exposure level. About 2.0% of administered dose was recovered in urine plus cage rinse and other collected label attributed to urine. About 0.1% of administered dose was found in feces. Most of the material balance was found in skin wash soap swabs or ethanol extracts of the swabs (>74% of administered dose), plus small additional amounts in the patch, patch securing materials, tape strips, and alcohol swabs. Thus absorption was determined to be about 2.1% of administered dose. Metabolism / Metabolites Metabolism in rats and ruminants involves cleavage of the ester, followed by hydroxylation of the cyclohexane ring. In rats, metabolism continues with cleavage of the enol ring, leading to formation of the cyclohexyl ester of 2,4-dichloromandelic acid, which is further metabolised. The residue definition comprises spirodiclofen enol. The primary initial product of ester cleavage (removing a 2,2-dimethylbutyric acid moiety) is designated BAJ 2510 (or BAJ 2740 enol). Two major products of this enol are the 4-OH and 3-OH addition products to the cyclohexyl ring, i.e. "4-OH BAJ 2510" and "3-OH BAJ 2510." Groups of Wistar Hsd/Cpb: Wu rats (about 200 g at treatment) were dosed with labeled spirodiclofen (radiopurity > 98%) in 10 mL/kg of 0.5% CMC suspension as follows (showing group designations in brackets): single high dose (100 mg/kg, 4 M); single low dose (including CO2 measurement) (2 mg/kg, 4 M); single low dose (EPA basic test) (2 mg/kg, 4 M); single low dose (2 mg/kg, 4 F); 14 daily doses with 2 mg/kg/day non-radioactive a.i., then 1 labeled low dose (2 mg/kg, 4 M); and single low dose, bile cannulation study (2 mg/kg, 6 M). ... There was a sex difference in urinary metabolites: low dose females excreted 53% of administered dose as the enol, whereas low dose males excreted low amounts of the enol (< 5%), but instead favored subsequent hydroxylation of the cyclohexyl moiety of the enol at carbon 3 or 4. Positions of the ring hydroxyls in the plain of the ring (designated "e" for equitorial) were most abundant: low dose males had 26% to 30% of administered label as the 3-hydroxy-enol (e) metabolite, and 13% to 15% of administered label as the 4-hydroxy-enol (e) metabolite. The associated axial "a" isomers with the hydroxyls perpendicular to the ring were comparatively minor metabolites. The combined 3- and 4-hydroxy-enol metabolites in females constituted only 17% of administered dose. There were no other common urinary metabolites. Pre-treatment with unlabeled low doses of spirodiclofen for 2 weeks had no obvious effect on metabolism. Fecal metabolism yielded 1 to 4% of parent spirodiclofen after low dose exposure, compared to 16% in high dose males (consistent with reduced absorption). The enol constituted 4 to 7% of administered dose in feces of low-dose non-cannulated rats (16% in high dose M), with 3- and 4-hydroxy-enol (e) metabolites as modest contributors (1% to 7% of administered dose for each of these isomers). Fecal metabolites included a few percent of mandelic acid-cyclohexyl-methyl esters (created by oxidatively opening the 5-membered ring at the location of the enol hydroxyl group), and subsequent metabolic products. Glucuronides were not observed in feces. The two most common bile residues were the OH-enol glucuronide and 3-hydroxy-enol (e) (3% and 4% of administered dose, respectively). At week 20, blood samples were taken from 4 dogs per sex at the high dose (600 ppm) at 0, 2, 4, 7, and 24 hours after feeding. Plasma concentrations of BAJ 2740 and the metabolite BAJ 2510 were evaluated by high performance liquid chromatography (HPLC). BAJ 2740 was below the limit of quantification since it was rapidly cleaved by esterases in plasma and liver to the metabolite BAJ 2510. No other metabolites were identified. Week 20 high dose group mean concentrations of metabolite BAJ 2510 in plasma were 24.8, 17.6, 19.1, 26.7, and 32.4 nmol/mL for males and 26.8, 15.9, 15.8, 25.0, and 28.1 nmol/mL in females at 0, 2, 4, 7, and 24 hours after feeding respectively. BAJ 2510 was also quantified in urine samples taken from 3 female and 1 male high dose (600 ppm) dogs at week 28. One hour after receiving treated diet, dogs were placed in metabolism cages for 5 hours. Urine volumes were 74, 281, and 305 mL in females and 18.6 mL in the male. BAJ 2510 concentrations in urine were 0.46, 0.16, and 0.12 umol/mL in females and 0.05 umol/mL in the male respectively. Biological Half-Life The half life of spirodiclofen was investigated using spiked rat plasma; it was estimated to be about 15 minutes. Groups of Wistar Hsd/Cpb: Wu rats (about 200 g at treatment) were dosed with labeled spirodiclofen (radiopurity > 98%) in 10 mL/kg of 0.5% CMC suspension as follows (showing group designations in brackets): single high dose (100 mg/kg, 4 M); single low dose (including CO2 measurement) (2 mg/kg, 4 M); single low dose (EPA basic test) (2 mg/kg, 4 M); single low dose (2 mg/kg, 4 F); 14 daily doses with 2 mg/kg/day non-radioactive a.i., then 1 labeled low dose (2 mg/kg, 4 M); and single low dose, bile cannulation study (2 mg/kg, 6 M). ... Plasma radioactivity typically dropped about 10-fold in all groups between 8 hr and 24 hr after dosing (plasma phase 1 elimination t 1/2 values were 2.4 hr to 4.2 hr). This is consistent with swift clearance from organs and tissues as previously reported. ... |
|---|---|
| 毒性/毒理 (Toxicokinetics/TK) |
Non-Human Toxicity Values
LC50 Rat inhalation >5000 mg/cu m/ 4 hr LD50 Rat dermal >2000 mg/kg. LD50 Rat oral >2500 mg/kg. |
| 参考文献 | |
| 其他信息 |
Spirodiclofen can cause cancer according to The Environmental Protection Agency (EPA).
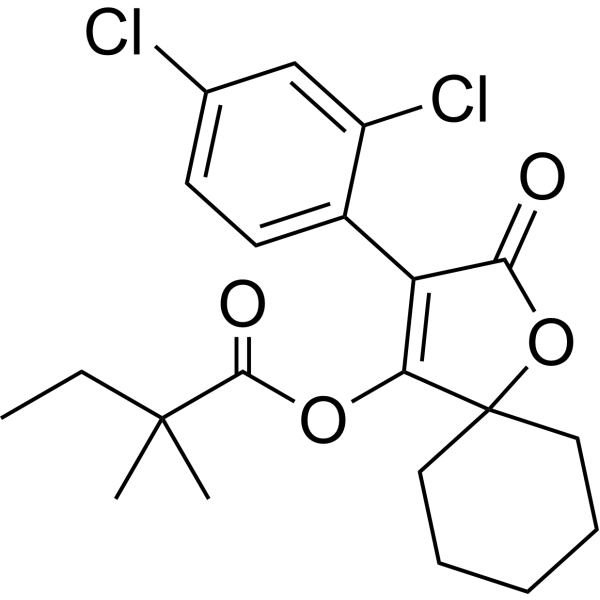
Spirodiclofen is an organochlorine acaricide, a dichlorobenzene, an oxaspiro compound and a gamma-lactone. It is functionally related to a 1,3-dichlorobenzene. Mechanism of Action Testicular mitochondrial preparations were evaluated for side chain cleavage of 25-OH cholesterol to pregnenolone (by assaying for progesterone after a subsequent oxidation step). In a mitochondrial preparation supplemented with NADP and in an environment of low malate levels (0.5 mM); 100 uM and 300 uM BAJ 2510 reduced progesterone synthesis to 68% and 24% of control groups, respectively. In contrast, spirodiclofen, 4-OH BAJ 2510, and 3-OH BAJ 2510 at concentrations up to 100 uM or (in the case of spirodiclofen, at the limits of solubility) had little or no effect on progesterone synthesis. When 0.5 mM citrate (and no malate) was present as a substrate (citrate also being capable of reducing NAD), even 300 uM BAJ 2510 had no remarkable effect on progesterone synthesis. This suggested an interference of BAJ 2510 with the Krebs cycle related to malate dehydrogenase activity. This was confirmed when investigators evaluated the oxidation of NADH due to malate dehydrogenase activity (assessing activity from both mitochondrial and cytoplasmic fractions): there was a clear dose-responsive inhibition of such activity due to BAJ 2510 concentrations in the 1 to 100 uM range (mitochondrial) or the 10 to 300 uM range (cytoplasmic). In contrast, BAJ 2510 had no effect on malic enzyme activity (assessed by NADP reduction with malate as substrate). In a dynamic organ culture of testicular tissue (6 hr incubation with steroidogenesis stimulated by 1 IU/mL hCG), BAJ 2510 concentrations of 10 to 300 uM caused marked, dose-related decrements in testosterone in both the tissue pieces and in the medium. An early step in progesterone synthesis from 25-OH cholesterol was markedly inhibited by BAJ 2510 in mitochondrial preparations. In contrast, progesterone levels were not statistically significantly reduced at any level with BAJ 2510 in the dynamic organ culture system with testicular tissue. As a positive control, ketoconazole profoundly reduced testosterone in tissues and medium, also without significantly reducing the quantity of progesterone in the tissue pieces. Thus it appears that BAJ 2510 toxicity is related to interference with cellular energy metabolism. /BAJ 2510, metabolite/ |
| 分子式 |
C21H24CL2O4
|
|---|---|
| 分子量 |
411.32
|
| 精确质量 |
410.105
|
| CAS号 |
148477-71-8
|
| PubChem CID |
177863
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.3±0.1 g/cm3
|
| 沸点 |
561.1±50.0 °C at 760 mmHg
|
| 熔点 |
94.8ºC
|
| 闪点 |
199.8±29.1 °C
|
| 蒸汽压 |
0.0±1.5 mmHg at 25°C
|
| 折射率 |
1.571
|
| LogP |
6.47
|
| tPSA |
52.6
|
| 氢键供体(HBD)数目 |
0
|
| 氢键受体(HBA)数目 |
4
|
| 可旋转键数目(RBC) |
5
|
| 重原子数目 |
27
|
| 分子复杂度/Complexity |
634
|
| 定义原子立体中心数目 |
0
|
| InChi Key |
DTDSAWVUFPGDMX-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C21H24Cl2O4/c1-4-20(2,3)19(25)26-17-16(14-9-8-13(22)12-15(14)23)18(24)27-21(17)10-6-5-7-11-21/h8-9,12H,4-7,10-11H2,1-3H3
|
| 化学名 |
[3-(2,4-dichlorophenyl)-2-oxo-1-oxaspiro[4.5]dec-3-en-4-yl] 2,2-dimethylbutanoate
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~120 mg/mL (~291.74 mM)
Ethanol : ~120 mg/mL (~291.74 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 3 mg/mL (7.29 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 30.0 mg/mL 澄清的 DMSO 储备液加入到400 μL PEG300中,混匀;再向上述溶液中加入50 μL Tween-80,混匀;然后加入450 μL 生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: 3 mg/mL (7.29 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 例如,若需制备1 mL的工作液,可将 100 μL 30.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 3 mg/mL (7.29 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: ≥ 3 mg/mL (7.29 mM) (饱和度未知) in 10% EtOH + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将100 μL 30.0 mg/mL 澄清乙醇储备液加入到 400 μL PEG300 中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 5 中的溶解度: 3 mg/mL (7.29 mM) in 10% EtOH + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 例如,若需制备1 mL的工作液,将 100 μL 30.0 mg/mL 澄清乙醇储备液加入 900 μL 20% SBE-β-CD 生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 配方 6 中的溶解度: ≥ 3 mg/mL (7.29 mM) (饱和度未知) in 10% EtOH + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 30.0 mg/mL 澄清乙醇储备液添加到 900 μL 玉米油中并充分混合。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.4312 mL | 12.1560 mL | 24.3120 mL | |
| 5 mM | 0.4862 mL | 2.4312 mL | 4.8624 mL | |
| 10 mM | 0.2431 mL | 1.2156 mL | 2.4312 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。