| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| Other Sizes |
|
| 靶点 |
CB2 receptor ( Ki = 0.6 nM )
|
|---|---|
| 体外研究 (In Vitro) |
SR144528 是一种有效的选择性 CB2 受体钳抗剂,Ki 为 0.6 nM。SR144528 单独能够以浓度依赖性方式(EC50=26±6 nM,两个实验)刺激 CHO-CB2 细胞中毛喉素敏感的腺苷酸环化酶有活性,在1 μM时效果最大(4倍刺激)而此时细胞浓度下,它对CHO-CB1没有显着影响(15% 抑制)[1]。辅以SR144528的原始264.7巨SR144528以浓度调节方式抑制磷脂体磷脂基辅酶A:磷脂基转移酶(ACAT)活性,IC50值为3.6±1.1 μM。在10 μM时,SR144528荧光约68%的ACAT活性[2]。
|
| 体内研究 (In Vivo) |
SR144528 在大脑 (10 mg/kg) 或 icv (10 μg/动物) 后,未观察到 [3H]-CP 55,940 与大脑大脑特定位点的结合。 SR144528 脾对大脑大麻素受体的参与时间依赖性,并且在粉末制剂3 mg/kg后至少18小时内显着[1]。 SR144528单独给药时不会对胃肠(GI)运动产生任何显着影响。SR144528不会爆发但会增强胃排空延迟[3]。
低镇痛剂量的WIN会延迟肠道转运,但高精神活性剂量则需要延迟胃排空。急性WIN对胃肠运动的影响仅限于给药后的最初几个小时。AM251部分抵消了WIN对胃肠运动的影响。令人惊讶的是,SR144528(而不是AM630)增强了WIN诱导的胃排空延迟。 结论与推论:X射线分析证实大麻素通过CB1受体抑制胃肠运动;此外,大麻素可以通过与SR144528敏感位点的相互作用影响运动。需要进一步的研究来验证这种作用位点是否是CB2受体[3]。 |
| 酶活实验 |
它测量 MAP 激酶的活性。简而言之,在应用配体前24小时,将已达到80%汇合的细胞保存在含有0.5%胎牛血清的培养基中。用 PBS 清洗后,将 CHO-CB1 或 -CB2 细胞在 37°C 下培养 20 分钟,无论是否存在 SR144528(10-9 至 3×10-6 M)。 4°C 洗涤后,将细胞在补充有 1% triton X-100、10 μg/mL 抑肽酶、10 μg/mL 亮肽素、1 mM 二硫苏糖醇和 1 mM 苯甲基磺酰氟的缓冲液 A 中裂解 15 分钟。缓冲液 A 含有 50 mM Tris-HCl,pH 7.5、150 mM NaCl、1 mM 乙二醇-双-(β-氨基乙基醚) N,N,N',N-四乙酸和 1 mM Na3PO<子>4。随后在 4°C 下以 14,000 xg 离心 15 分钟分离已溶解的细胞提取物。使用前,取出 15 μL 等分试样并将其储存在 -80°C 下。使用 γ-[33P]ATP 和 p42/p44 MAP 激酶系统,磷酸化测定在 30°C 下运行 30 分钟(线性测定条件)。通过使用液体闪烁计数,可以确定所包含的放射性[1]。
|
| 细胞实验 |
在 CHO-CB1 或 -CB2 细胞中,进行 cAMP 积累。使用 PBS 清洗细胞,然后将细胞在 1 mL PBS 中于 37°C 下孵育 15 分钟,无论是否含有 SR144528(3×10-9 至 10-5 M)在场。接下来,细胞在 37°C 下孵育 20 分钟,并添加毛喉素(3 μM 最终浓度)。快速吸出测定介质后,加入 1.5 mL 冰冷的 50 mM Tris-HCl(pH 8)和 4 mM 乙二胺四乙酸以终止反应。将培养皿在冰上放置五分钟后,将提取物移至玻璃管中。提取物经过煮沸和 3500 g 离心 10 分钟,以去除任何残留的细胞碎片。 cAMP 的浓度通过放射免疫测定法使用来自已干燥的上清液的等分试样和闪烁邻近测定系统来测量。如果没有毛喉素,则计算基础活性[1]。
|
| 动物实验 |
In this study, male Wistar rats weighing between 240 and 300 grams are utilized. Three distinct sets of experiments are conducted a week after the animals were brought to the lab. Rats receive an intraperitoneal injection of SR144528 (1 mg/kg) in the third series of experiments. Rats given vehicles are also used to examine the impact of SR144528. The maximum volume for SR144528 is set at 4 to 5 mL/kg[3].
Male Wistar rats received different doses of WIN and both psychoactivity (cannabinoid tetrad) and GI motility (radiographic analysis) were tested. The duration of WIN effect on GI motility was also radiographically analyzed. Finally, the involvement of the different cannabinoid receptors on WIN-induced alterations of GI motility was analyzed by the previous administration of selective CB1 (AM251) and CB2 (SR144528 or AM630) antagonists. After administration of contrast medium, alterations in GI motility were quantitatively evaluated in serial radiographs by assigning a compounded value to each region of the GI tract. [3] |
| 参考文献 |
|
| 其他信息 |
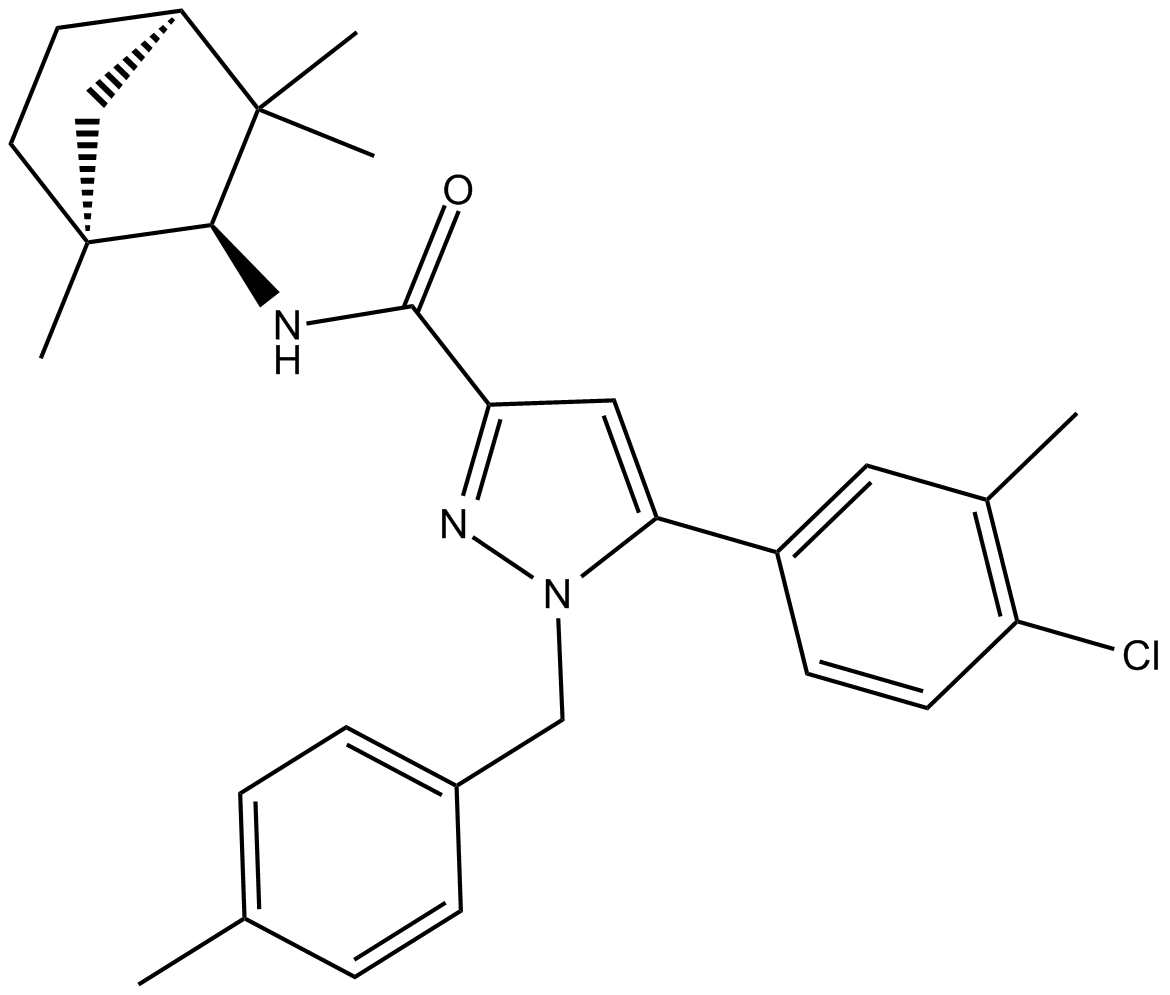
SR 144528 is a secondary carboxamide resulting from the formal condensation of the carboxy group of 5-(4-chloro-3-methylphenyl)-1-(4-methylbenzyl)-1H-pyrazole-3-carboxylic acid with the amino group of (1S,2S,4R)-1,3,3-trimethylbicyclo[2.2.1]heptan-2-amine. A potent and selective cannabinoid receptor type 2 (CB2 receptor) inverse agonist (Ki = 0.6 nM). It has a role as a CB2 receptor antagonist and an EC 2.3.1.26 (sterol O-acyltransferase) inhibitor. It is a member of pyrazoles, a secondary carboxamide, a member of monochlorobenzenes and a bridged compound.
Based on both binding and functional data, this study introduces SR 144528 as the first, highly potent, selective and orally active antagonist for the CB2 receptor. This compound which displays subnanomolar affinity (Ki = 0.6 nM) for both the rat spleen and cloned human CB2 receptors has a 700-fold lower affinity (Ki = 400 nM) for both the rat brain and cloned human CB1 receptors. Furthermore it shows no affinity for any of the more than 70 receptors, ion channels or enzymes investigated (IC50 > 10 microM). In vitro, SR 144528 antagonizes the inhibitory effects of the cannabinoid receptor agonist CP 55,940 on forskolin-stimulated adenylyl cyclase activity in cell lines permanently expressing the h CB2 receptor (EC50 = 10 nM) but not in cells expressing the h CB1 (no effect at 10 microM). Furthermore, SR 144528 is able to selectively block the mitogen-activated protein kinase activity induced by CP 55,940 in cell lines expressing h CB2 (IC50 = 39 nM) whereas in cells expressing h CB1 an IC50 value of more than 1 microM is found. In addition, SR 144528 is shown to antagonize the stimulating effects of CP 55,940 on human tonsillar B-cell activation evoked by cross-linking of surface Igs (IC50 = 20 nM). In vivo, after oral administration SR 144528 totally displaced the ex vivo [3H]-CP 55,940 binding to mouse spleen membranes (ED50 = 0.35 mg/kg) with a long duration of action. In contrast, after the oral route it does not interact with the cannabinoid receptor expressed in the mouse brain (CB1). It is expected that SR 144528 will provide a powerful tool to investigate the in vivo functions of the cannabinoid system in the immune response. [1] Oxysterol-induced macrophage apoptosis may have a role in atherosclerosis. Macrophages lacking the type 2 cannabinoid receptor (CB2) are partially resistant to apoptosis induced by 7-ketocholesterol (7KC). AM-251 and SR144528 are selective antagonists of CB1 and CB2 receptors, respectively. We observed that both compounds reduce 7KC-induced apoptosis in Raw 264.7 macrophages. As oxysterol-induced macrophage apoptosis requires acyl-coenzymeA:cholesterol acyltransferase (ACAT) activity, we tested their affects on ACAT activity. AM-251 and SR144528 both reduced cholesteryl ester synthesis in unstimulated and acetylated LDL-stimulated Raw 264.7 macrophages, CB2(+/+) and CB2(-/-) peritoneal macrophages, as well as in vitro, in mouse liver microsomes. Consistent with inhibition of ACAT, the development of foam cell characteristics in macrophages by treatment with acetylated LDL was reduced by both compounds. This work is the first evidence that AM-251 and SR144528 are inhibitors of ACAT and as a result, might have anti-atherosclerotic activities independent of their affect on cannabinoid signaling. [2] |
| 分子式 |
C29H34CLN3O
|
|---|---|
| 分子量 |
476.06
|
| 精确质量 |
475.239
|
| 元素分析 |
C, 73.17; H, 7.20; Cl, 7.45; N, 8.83; O, 3.36
|
| CAS号 |
192703-06-3
|
| 相关CAS号 |
192703-06-3
|
| PubChem CID |
3081355
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.2±0.1 g/cm3
|
| 沸点 |
627.7±55.0 °C at 760 mmHg
|
| 闪点 |
333.4±31.5 °C
|
| 蒸汽压 |
0.0±1.8 mmHg at 25°C
|
| 折射率 |
1.633
|
| LogP |
7.05
|
| tPSA |
46.92
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
2
|
| 可旋转键数目(RBC) |
5
|
| 重原子数目 |
34
|
| 分子复杂度/Complexity |
750
|
| 定义原子立体中心数目 |
3
|
| SMILES |
O=C(C1=NN(CC2=CC=C(C)C=C2)C(C3=CC=C(Cl)C(C)=C3)=C1)N[C@H]4[C@@](C5)(C)CC[C@@]5([H])C4(C)C
|
| InChi Key |
SUGVYNSRNKFXQM-XRHWURSXSA-N
|
| InChi Code |
InChI=1S/C29H34ClN3O/c1-18-6-8-20(9-7-18)17-33-25(21-10-11-23(30)19(2)14-21)15-24(32-33)26(34)31-27-28(3,4)22-12-13-29(27,5)16-22/h6-11,14-15,22,27H,12-13,16-17H2,1-5H3,(H,31,34)/t22-,27-,29+/m1/s1
|
| 化学名 |
5-(4-chloro-3-methylphenyl)-1-[(4-methylphenyl)methyl]-N-[(1S,2S,4R)-1,3,3-trimethyl-2-bicyclo[2.2.1]heptanyl]pyrazole-3-carboxamide
|
| 别名 |
SR-144528; SR 144528; 192703-06-3; CHEMBL381791; 1H-Pyrazole-3-carboxamide, 5-(4-chloro-3-methylphenyl)-1-[(4-methylphenyl)methyl]-N-[(1S,2S,4R)-1,3,3-trimethylbicyclo[2.2.1]hept-2-yl]-; SR144,528; 5-(4-chloro-3-methylphenyl)-1-(4-methylbenzyl)-N-((1S,2S,4R)-1,3,3-trimethylbicyclo[2.2.1]heptan-2-yl)-1H-pyrazole-3-carboxamide; SR144528
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: ~50 mg/mL (~105.0 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (5.25 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (5.25 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL 澄清 DMSO 储备液加入到 900 μL 玉米油中并混合均匀。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.1006 mL | 10.5029 mL | 21.0058 mL | |
| 5 mM | 0.4201 mL | 2.1006 mL | 4.2012 mL | |
| 10 mM | 0.2101 mL | 1.0503 mL | 2.1006 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
|
|
|