| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
SRPK1/serine arginine protein kinase 1 (Ki = 0.89 μM)
SRPIN340 (SRPK inhibitor) specifically targets serine-arginine protein kinase 1 (SRPK1) with an IC50 of 0.8 μM [1] SRPIN340 (SRPK inhibitor) inhibits SRPK2 with an IC50 of 2.3 μM, and shows no significant inhibition of other kinases (e.g., CDK1, CDK2, ERK1, JNK1) at concentrations up to 10 μM [1] |
|---|---|
| 体外研究 (In Vitro) |
SRPIN340 在 Flp-In293 细胞中抑制 SRPK 的 SR 磷酸化,并以剂量依赖性方式促进 SRp75 降解,从而抑制 HIV 产生。 SRPIN340 剂量 (5 mg/mL) 不会导致 CHO 细胞的染色体结构和染色体数量出现异常。 SRPIN340 在体外以剂量依赖性方式抑制 HCV 亚基因组复制子的表达和 HCV-JFH1 克隆的复制。细胞分析:SRPIN340 已被证明可以抑制 HIV-1 和其他需要 SR 蛋白依赖性 RNA 加工才能在 HIV-1 转染或感染的 Flp-In293 细胞系中繁殖的病毒的复制。此外,据报道,SRPIN340 可显着抑制 SRPK1 和 SRPK2 激酶活性,但不会有效抑制其他 SRPK,例如 Clk1 和 Clk4,SRPK1 的 Ki 值为 0.89μM。此外,SRPIN340 已被证明可以剂量依赖性地促进 SRp75 的降解,而 SRp75 是 HIV 表达所必需的。此外,SRPIN340 显示出对 Sindbis 病毒增殖的抑制作用,IC50 为 60μM,并能防止 Sindbis 病毒的细胞病变作用。
在 HeLa 细胞中,SRPIN340 (SRPK抑制剂)(1–10 μM)剂量依赖性抑制 SRPK1 介导的丝氨酸-精氨酸(SR)蛋白(如 ASF/SF2)磷酸化。5 μM 时,它阻断纤连蛋白(fibronectin)和 CD44 前体 mRNA 的可变剪接,使剪接模式向非致癌异构体转变 [1] - 针对耐甲氧西林金黄色葡萄球菌(MRSA)和耐万古霉素肠球菌(VRE)的临床分离株,SRPIN340 (SRPK抑制剂) 表现出抗菌活性,最低抑菌浓度(MIC)分别为 8–16 μg/mL(MRSA)和 16–32 μg/mL(VRE)。8 μg/mL 时抑制细菌生物膜形成约 50%,16 μg/mL 时减少细菌对人上皮细胞的黏附约 60% [2] - 在过氧化氢(H2O2)处理的人视网膜色素上皮(RPE)细胞中,SRPIN340 (SRPK抑制剂)(5–20 μM)对氧化应激诱导的细胞死亡具有保护作用:20 μM 时细胞活力从 42% 提升至 78%。它减少细胞内活性氧(ROS)生成约 45%,抑制 caspase-3 激活(20 μM 时约 55%)[3] - 在人癌细胞系(HeLa、MCF-7、A549、HCT116)中,SRPIN340 (SRPK抑制剂) 抑制细胞增殖,72 小时 IC50 值分别为 HeLa(3.2 μM)、MCF-7(4.1 μM)、A549(4.8 μM)、HCT116(5.3 μM)。它诱导 G2/M 期细胞周期阻滞和凋亡:5 μM 时,凋亡率分别为 HeLa(~35%)、MCF-7(~30%)、A549(~28%)、HCT116(~25%)。Western blot 显示 Bax/Bcl-2 比值和 cleaved caspase-3 上调,cyclin B1 和 CDK1 下调 [4] - SRPIN340 (SRPK抑制剂)(2–10 μM)改变 HeLa 细胞中癌症相关基因(如 Bcl-x、survivin)的可变剪接,5 μM 时使促凋亡 Bcl-xS 异构体增加,抗凋亡 Bcl-xL 异构体减少约 40% [4] |
| 体内研究 (In Vivo) |
SRPIN340 在体内以剂量依赖性方式抑制 CNV 形成。 SRPIN340 显着降低 VEGF、MCP-1、ICAM-1 的蛋白水平,从而抑制巨噬细胞浸润。
为了确定SRPK阻断是否抑制CNV的形成,我们量化了服用或不服用SRPIN340的RPE脉络膜复合物平片中的CNV大小。激光损伤后7天,与赋形剂治疗的动物(30737±3758μm2,n=31,p<0.05;图2A,B)相比,用2 pmolSRPIN340治疗的动物的平均CNV大小(19870±1935μm2)显著减小(n=33;n表示CNV损伤的数量)。此外,与2 pmol SRPIN340治疗的动物相比,更高剂量的SRPIN340给药(20 pmol;n=23)显著降低了CNV大小(15649±1803μm2,p<0.01),而较低剂量的给药(0.2 pmol;n=17)没有显著抑制CNV的形成(21741±3695μm2,p=0.10;图2A,B)。单独接受激光损伤的小鼠与接受激光和玻璃体内注射赋形剂溶液的小鼠之间,CNV大小没有显著差异。数据表明,SRPK阻断以剂量依赖的方式抑制CNV生长。[3] 为了研究SRPIN340对Vegf亚型的影响,使用实时PCR分析了总Vegf和含外显子8a的Vegf亚基的mRNA表达。与用0.1%DMSO处理的小鼠(n=8;n表示眼睛数量)相比,用20pmol SRPIN340(n=6)处理的小鼠RPE脉络膜复合物中总Vegf的mRNA表达显著降低了56%(p<0.05;图3A)。同样,含Vegf外显子8a的mRNA表达显著降低了57%(p<0.05;图3B)。此外,用20pmol SRPIN340治疗的小鼠的总VEGF浓度(209.2±10.9 pg/mg,n=8)明显低于用0.1%DMSO治疗的小鼠(274.2±17.9 pg/mg)(n=10,p<0.01;图3C)。[3] SRPIN340以30 mg ml−1的浓度溶解在丙二醇(80%)和DMSO(20%)(但不是20%DMSO/80%水)中。通过卵圆管饲法以单次100μl剂量(100 mg kg−1)给药,并对血液和组织进行取样和质谱分析。在血浆中,1小时后检测到SRPIN340的浓度为1.55±0.91μg ml−1,4小时和8小时后分别降至0.43±0.19和0.77±0.2μg ml-1。24小时后,SRPIN340的血浆浓度为0.2±0.06μg ml−1。进行了单相指数衰减曲线拟合,SRPIN340在血浆中的持续半衰期(从1小时开始拟合的曲线)为13.49小时。然而,在递送的3 mg SRPIN中,血浆SRPIN340总量估计为2.0μg(30 g小鼠,45%红细胞压积,80 ml kg−1血容量),而胃中的浓度为100μg ml-1,表明药物吸收不良(补充图1)。此外,全身给药需要高浓度的DMSO(20%)。因此,我们尝试在体内局部注射SRPIN340以避免全身治疗。将未转导的A375细胞皮下注射并使其形成肿瘤。与DMSO(1%)对照注射的肿瘤相比,在靠近肿瘤部位的100μl 1×PBS中每天皮下注射2μg SRPIN340可显著降低肿瘤生长(P<0.001;单因素方差分析-Bonferroni事后;图5C)。肿瘤后分析显示,SRPIN340治疗的肿瘤中VEGF总表达降低(P<0.05,Student非配对t检验),抗血管生成VEGFxxxb亚型的检测没有差异,似乎不受治疗的影响(图5D)。在这项研究中,与敲除不同,肿瘤的大小足以切片和染色CD31作为微血管密度(MVD)的指标。与载体治疗的肿瘤相比,SRPIN340显著降低了MVD(图5E)。 在 HeLa 宫颈癌裸鼠异种移植模型中,腹腔注射 SRPIN340 (SRPK抑制剂)(15 mg/kg,每周 2 次,持续 4 周)显著抑制肿瘤生长。与溶媒对照组相比,肿瘤体积减少约 58%,肿瘤重量减轻约 52%。免疫组化染色显示,肿瘤组织中 Ki-67 增殖指数从 ~70% 降至 ~32%,TUNEL 阳性凋亡细胞从 ~5% 增至 ~28% [4] - 在小鼠视网膜光损伤模型中,光暴露前 24 小时玻璃体内注射 SRPIN340 (SRPK抑制剂)(5 μg/眼)减轻视网膜功能障碍。视网膜电图(ERG)分析显示,a 波和 b 波振幅分别较溶媒对照组增加约 42% 和 38%。组织学检查显示,外核层光感受器细胞损失减少约 45% [3] |
| 酶活实验 |
体外激酶抑制活性和动力学分析。[1]
His6标记的mSRPK1、mSRPK2、mClk1和mClk4在大肠杆菌(BL21)中表达,并按所述纯化。酶和底物与指定浓度的ATP和SRPIN340一起孵育(如图3所示)。如所述测量SRPK1激酶活性。 使用SigmaPlot软件通过Lineweaver–Burk Plot分析抑制活性。[1] 酶联免疫吸附试验[3] 每只眼睛放置四个激光损伤,并玻璃体内注射1μl 0.1%DMSO或SRPIN340。根据制造商的方案,使用酶联免疫吸附测定试剂盒测定上清液中VEGF、单核细胞趋化蛋白(MCP)-1和细胞间粘附分子(ICAM)-1的蛋白质水平,并将其标准化为总蛋白。 SRPK1 激酶活性实验:重组人 SRPK1 蛋白与源自 ASF/SF2 的合成肽底物(含 SRPK 磷酸化位点)在激酶缓冲液中孵育。将系列稀释(0.01–10 μM)的 SRPIN340 (SRPK抑制剂) 加入反应体系,随后加入 [γ-32P]ATP。30°C 孵育 30 分钟后,将混合物点样到磷酸纤维素纸上,洗去未结合的放射性物质,通过液体闪烁计数法测量结合底物的放射性强度,计算抑制率和 IC50 值 [1] - SRPK2 激酶活性实验:采用与 SRPK1 实验相同的流程,以重组人 SRPK2 蛋白为酶,通过测量肽底物磷酸化的抑制程度确定 IC50 值 [1] - 激酶选择性实验:在 20 种不同激酶(包括 CDK1、CDK2、ERK1、JNK1、PKCα 等)中测试 SRPIN340 (SRPK抑制剂)(10 μM)的抑制活性。采用放射性实验方法检测激酶活性,计算每种激酶的抑制率以评估选择性 [1] |
| 细胞实验 |
在 96 孔板中,接种白血病细胞(5 × 10 4 细胞/孔)和分离的 PBMC(8 × 10 4 细胞/孔)。每个孔装有 100 μL 完整 RPMI 培养基和 100 μL SRPIN340 溶液,其浓度各不相同。将 10% 胎牛血清和 0.4% DMSO (v/v) 添加到 RPMI 培养基中以稀释化合物。培养 48 小时(3 小时,37°C)后,将 MTT (5 mg/mL) 添加到孔中。室温下 30 分钟并以 500 × g 离心后,从板中除去 MTT 溶液,并添加 100 μL/孔的 DMSO 以溶解甲臜。使用酶标仪测量 540 nm 处的吸光度。每个实验方案都运行三次[2]。
细胞活力测定细胞增殖通过两种方法测定。将每孔30000个A375细胞接种在24孔板上,这些细胞用打乱的shRNA、SRPK1-shRNA转导或未转导,并用SRPIN340处理。每24小时对细胞进行胰蛋白酶处理,并进行细胞计数。接种在盖玻片上的细胞也被Ki67染色。对于划痕试验,细胞在24孔板中生长至融合,并沿孔的中心线从板上刮下1mm厚的细胞线。在时间零点、12小时后和24小时后对每个孔进行成像。将划痕的覆盖百分比确定为伤口闭合百分比的衡量标准。[4] SR 蛋白磷酸化实验:HeLa 细胞血清饥饿 12 小时,用 SRPIN340 (SRPK抑制剂)(1–10 μM)处理 4 小时。制备细胞裂解液,通过 Western blot 用磷酸化特异性抗体检测磷酸化 SR 蛋白(ASF/SF2),总 ASF/SF2 作为上样对照 [1] - 可变剪接分析:HeLa 细胞用 SRPIN340 (SRPK抑制剂)(2–10 μM)处理 24 小时,提取总 RNA 并逆转录为 cDNA,使用针对纤连蛋白、CD44、Bcl-x 或 survivin 前体 mRNA 的特异性引物进行 RT-PCR。剪接异构体通过琼脂糖凝胶电泳分离,光密度法定量 [1,4] - 抗菌细胞实验:MRSA 或 VRE 菌株在 Mueller-Hinton 肉汤中培养至对数中期,加入 SRPIN340 (SRPK抑制剂)(0.5–64 μg/mL),37°C 孵育 24 小时。通过梯度稀释和平板接种测定细菌活力,最低抑菌浓度(MIC)定义为抑制细菌可见生长的最低浓度。96 孔板中细菌培养物经结晶紫染色评估生物膜形成 [2] - RPE 细胞氧化应激实验:人 RPE 细胞接种到 96 孔板培养至汇合,用 SRPIN340 (SRPK抑制剂)(5–20 μM)预处理 1 小时,再用 H2O2(200 μM)处理 24 小时。CCK-8 法检测细胞活力,DCFH-DA 荧光探针检测 ROS 生成,比色法试剂盒检测 caspase-3 活性 [3] - 癌细胞增殖和凋亡实验:癌细胞(HeLa、MCF-7、A549、HCT116)以 5×103 个细胞/孔接种到 96 孔板,用 SRPIN340 (SRPK抑制剂)(0.5–20 μM)处理 72 小时。MTT 法评估细胞活力以计算 IC50 值。细胞周期分析中,5 μM SRPIN340 (SRPK抑制剂) 处理细胞 24 小时,乙醇固定,碘化丙啶染色,流式细胞术分析。Annexin V-FITC/PI 染色和 Western blot 检测 Bax、Bcl-2、cleaved caspase-3 评估凋亡 [4] |
| 动物实验 |
mouse model with choroidal neovascularization (CNV)
~20 pmol i.v. SRPIN340 (50 mM in 100% dimethyl sulfoxide, DMSO) was diluted with phosphate buffered saline (PBS, potassium chloride, 2.68 mM; potassium phosphate monobasic, 1.47 mM; sodium chloride, 136.89 mM; sodium phosphate dibasic, 8.10 mM) to various concentrations in 0.1% DMSO before treatment. Mice were divided into five groups: CNV induction alone (the control group) and CNV induction with 1 μl intravitreal injection of either 0.1% DMSO, 0.2 pmol, 2 pmol, or 20 pmol SRPIN340. Intravitreal injection was performed using a 33-gauge needle immediately after laser photocoagulation.[3] In vivo tumour model All animal experiments were carried out under a UK Home Office License after approval by the University of Bristol Ethical Review Group. A375, A375 shRNA control and A375 shRNA SRPK1 knockdown cells were cultured in T75 flasks to 80% confluence. Trypsinised cells were counted using a haemocytometer, and 2 million cells of A375 shRNA control and A375 shRNA SRPK1 were injected subcutaneously either into the left and right flanks of nude mice, or a single injection of untransduced A375 cells. Tumour-bearing mice (>3 mm) were weighed and tumours were measured by caliper bi-weekly. Mice bearing A375-untransfected tumours were treated with either 100 μl of 20 μg ml−1 SRPIN340 (diluted 100 × in PBS from 2 mg ml−1 stock in DMSO), or 100 μl of 1% DMSO vehicle control injected daily into the peritumoral space. [4] HeLa xenograft nude mouse model: Female BALB/c nude mice (6–8 weeks old) were subcutaneously injected with HeLa cells (5×106 cells/mouse) into the right flank. When tumors reached a volume of ~100 mm³, mice were randomly divided into control and treatment groups (n=6/group). SRPIN340 (SRPK inhibitor) was dissolved in DMSO and diluted with normal saline (final DMSO concentration ≤5%), then administered intraperitoneally at 15 mg/kg twice weekly for 4 weeks. Control mice received vehicle (DMSO/saline). Tumor volume (measured by caliper every 3 days) and body weight (measured weekly) were recorded. At the end of the experiment, mice were sacrificed, tumors were excised, weighed, and fixed in formalin for immunohistochemical analysis (Ki-67 and TUNEL staining) [4] - Retinal light damage mouse model: Male C57BL/6 mice (8–10 weeks old) were dark-adapted for 24 hours. SRPIN340 (SRPK inhibitor) was dissolved in sterile phosphate-buffered saline (PBS) to a concentration of 1 μg/μL. Intravitreal injection of 5 μL SRPIN340 (SRPK inhibitor) (5 μg/eye) was performed under anesthesia. Control mice received 5 μL PBS. Twenty-four hours after injection, mice were exposed to white light (10,000 lux) for 2 hours. Seven days later, ERG was performed to evaluate retinal function, and mice were sacrificed to collect eyes for histological analysis of the outer nuclear layer [3] |
| 毒性/毒理 (Toxicokinetics/TK) |
In vitro toxicity: SRPIN340 (SRPK inhibitor) (0.5–20 μM) did not affect the viability of normal human foreskin fibroblasts (NHF) or primary retinal cells, with cell viability maintained above 85% at all tested concentrations [3,4]
- In vivo toxicity: In the HeLa xenograft model, intraperitoneal administration of SRPIN340 (SRPK inhibitor) (15 mg/kg, twice weekly for 4 weeks) did not cause significant changes in mouse body weight (control vs. treatment: ~20 g vs. ~19.2 g) or obvious toxic symptoms (e.g., lethargy, loss of appetite, organ abnormalities). Serum ALT, AST, creatinine, and urea nitrogen levels were within normal ranges [4] - In the retinal light damage model, intravitreal injection of SRPIN340 (SRPK inhibitor) (5 μg/eye) did not induce intraocular inflammation or structural damage to the retina, as shown by histological examination [3] |
| 参考文献 | |
| 其他信息 |
Although the viral genome is often quite small, it encodes a broad series of proteins. The virus takes advantage of the host-RNA-processing machinery to provide the alternative splicing capability necessary for the expression of this proteomic diversity. Serine-arginine-rich (SR) proteins and the kinases that activate them are central to this alternative splicing machinery. In studies reported here, we use the HIV genome as a model. We show that HIV expression decreases overall SR protein/activity. However, we also show that HIV expression is significantly increased (20-fold) when one of the SR proteins, SRp75 is phosphorylated by SR protein kinase (SRPK)2. Thus, inhibitors of SRPK2 and perhaps of functionally related kinases, such as SRPK1, could be useful antiviral agents. Here, we develop this hypothesis and show that HIV expression down-regulates SR proteins in Flp-In293 cells, resulting in only low-level HIV expression in these cells. However, increasing SRPK2 function up-regulates HIV expression. In addition, we introduce SR protein phosphorylation inhibitor 340 (SRPIN340), which preferentially inhibits SRPK1 and SRPK2 and down-regulates SRp75. Although an isonicotinamide compound, SPRIN340 (or its derivatives) remain to be optimized for better specificity and lower cytotoxicity, we show here that SRPIN340 suppresses propagation of Sindbis virus in plaque assay and variably suppresses HIV production. Thus, we show that SRPK, a well known kinase in the cellular RNA-processing machinery, is used by at least some viruses for propagation and hence suggest that SRPIN340 or its derivatives may be useful for curbing viral diseases.[1]
Splicing of messenger RNAs is regulated by site-specific binding of members of the serine-arginine-rich (SR) protein family, and SR protein kinases (SRPK) 1 and 2 regulate overall activity of the SR proteins by phosphorylation of their RS domains. We have reported that specifically designed SRPK inhibitors suppressed effectively several DNA and RNA viruses in vitro and in vivo. Here, we show that an SRPK inhibitor, SRPIN340, suppressed in a dose-dependent fashion expression of a hepatitis C virus (HCV) subgenomic replicon and replication of the HCV-JFH1 clone in vitro. The inhibitory effects were not associated with antiproliferative or nonspecific cytotoxic effects on the host cells. Overexpression of SRPK1 or SRPK2 resulted in augmentation of HCV replication, while small interfering RNA (siRNA) knockdown of the SRPKs suppressed HCV replication significantly. Immunocytochemistry showed that SRPKs and the HCV core and NS5A proteins colocalized to some extent in the perinuclear area. Our results demonstrate that SRPKs are host factors essential for HCV replication and that functional inhibitors of these kinases may constitute a new class of antiviral agents against HCV infection.[2] Purpose: To investigate the applicability of serine/arginine-rich protein kinase (SRPK)-specific inhibitor, SRPIN340, for attenuation of choroidal neovascularization (CNV) formation using a mouse model.[3] Methods: Laser photocoagulation was performed to induce CNV in C57BL/6J mice, followed by intravitreal injection of SRPIN340 or vehicle. Seven days after the treatment, the CNV size was evaluated using a flatmount technique. Protein levels of vascular endothelial growth factor (VEGF) and inflammation-associated molecules, such as monocyte chemoattractant protein (MCP)-1 and intercellular adhesion molecule (ICAM)-1, in the retinal pigment epithelium-choroid complex were measured with enzyme-linked immunosorbent assay. Expression levels of total Vegf, exon 8a-containing Vegf isoforms, and F4/80 (a specific marker for macrophage) were assessed using real-time PCR.[3] Results: SRPIN340 inhibited CNV formation in a dose-dependent manner. Compared with the vehicle, SRPIN340 significantly decreased the protein levels of VEGF, MCP-1, ICAM-1, and consequently inhibited macrophage infiltration. Furthermore, SRPIN340 suppressed the gene expression levels of total Vegf and exon 8a-containing Vegf isoforms.[3] Conclusions: SRPIN340, a specific inhibitor of SRPK, suppressed Vegf expression and attenuated CNV formation. Our data suggest the possibility that SRPIN340 is applicable for neovascular age-related macular degeneration as a novel chemical therapeutics.[3] SRPIN340 (SRPK inhibitor) is a selective small-molecule inhibitor of SRPK1 and SRPK2, identified through a high-throughput screen for compounds that block SRPK-mediated SR protein phosphorylation [1] - Its core mechanism of action involves inhibition of SRPK-catalyzed SR protein phosphorylation, which regulates alternative RNA splicing of genes involved in cell proliferation, apoptosis, and other biological processes [1,4] - SRPIN340 (SRPK inhibitor) exhibits antibacterial activity against drug-resistant Gram-positive bacteria (MRSA, VRE) by inhibiting biofilm formation and bacterial adherence [2] - It exerts neuroprotective effects in retinal light damage by reducing oxidative stress and caspase-dependent apoptosis [3] - The antitumor activity of SRPIN340 (SRPK inhibitor) is associated with G2/M cell cycle arrest, induction of apoptosis, and modulation of alternative splicing of cancer-related genes [4] - SRPIN340 (SRPK inhibitor) shows potential therapeutic applications in cancer, drug-resistant bacterial infections, and retinal degenerative diseases [1,2,3,4] |
| 分子式 |
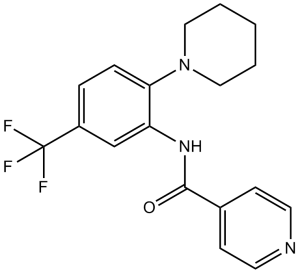
C18H18F3N3O
|
|
|---|---|---|
| 分子量 |
349.35
|
|
| 精确质量 |
349.14
|
|
| 元素分析 |
C, 61.88; H, 5.19; F, 16.31; N, 12.03; O, 4.58
|
|
| CAS号 |
218156-96-8
|
|
| 相关CAS号 |
|
|
| PubChem CID |
2797577
|
|
| 外观&性状 |
White to light yellow solid powder
|
|
| 密度 |
1.3±0.1 g/cm3
|
|
| 沸点 |
395.9±42.0 °C at 760 mmHg
|
|
| 闪点 |
193.3±27.9 °C
|
|
| 蒸汽压 |
0.0±0.9 mmHg at 25°C
|
|
| 折射率 |
1.578
|
|
| LogP |
4.15
|
|
| tPSA |
45.23
|
|
| 氢键供体(HBD)数目 |
1
|
|
| 氢键受体(HBA)数目 |
6
|
|
| 可旋转键数目(RBC) |
3
|
|
| 重原子数目 |
25
|
|
| 分子复杂度/Complexity |
445
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
FC(C1C([H])=C([H])C(=C(C=1[H])N([H])C(C1C([H])=C([H])N=C([H])C=1[H])=O)N1C([H])([H])C([H])([H])C([H])([H])C([H])([H])C1([H])[H])(F)F
|
|
| InChi Key |
DWFGGOFPIISJIT-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C18H18F3N3O/c19-18(20,21)14-4-5-16(24-10-2-1-3-11-24)15(12-14)23-17(25)13-6-8-22-9-7-13/h4-9,12H,1-3,10-11H2,(H,23,25)
|
|
| 化学名 |
N-[2-piperidin-1-yl-5-(trifluoromethyl)phenyl]pyridine-4-carboxamide
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (7.16 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 配方 2 中的溶解度: 1% DMSO +30% polyethylene glycol+1% Tween 80 : 8 mg/mL 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.8625 mL | 14.3123 mL | 28.6246 mL | |
| 5 mM | 0.5725 mL | 2.8625 mL | 5.7249 mL | |
| 10 mM | 0.2862 mL | 1.4312 mL | 2.8625 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
|
|---|
|
|