| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 5g |
|
||
| Other Sizes |
|
| 靶点 |
Retinoic acid receptor α (RARα)
|
|---|---|
| 体外研究 (In Vitro) |
在 T 细胞淋巴瘤细胞中,他米巴罗汀 (20, 40 μM) 抑制与细胞周期相关的基因的表达。当将他米巴罗汀 (5 μM) 添加到 RARA 过表达细胞中时,与 RARAlow 细胞相比,它显着增强了 RARE 活性。此外,由于 RARAwt 过度表达,用他米巴罗汀治疗可增强 CDK2、CDK4 和 CDK6 的抑制程度 [1]。他米巴罗汀直接抵消经转化生长因子-β1 处理的真皮成纤维细胞的促纤维化表型,抑制内皮细胞中 ICAM-1 的表达,并在体外刺激 M1 巨噬细胞的发育 [2]。在 VSMC 中,他米巴罗汀 (4 μM) 剂量依赖性地增加 apelin mRNA 和蛋白质水平。由于与预先结合到 apelin 启动子的 TCE 位点的 KLF5 和 Sp1 相互作用,RARα(视黄酸受体 α)在他米巴罗汀刺激期间被招募到 apelin 启动子。然后,该转录激活复合物触发 apelin 表达。 VSMC 升高。通过直接与 apelin 启动子上的 TCE 结合,KLF5 和 Sp1 共同介导他米巴罗汀诱导的 apelin 表达 [4]。
Am80抑制CD4+T细胞中IL-4、IL-17A和IFN-γ的产生。 Am80抑制巨噬细胞的M2极化。 Am80降低真皮微血管内皮细胞ICAM-1的表达。 Am80直接改善TGF-β诱导的人皮肤成纤维细胞纤维化表型[2]。 |
| 体内研究 (In Vivo) |
在用博来霉素 (BLM) 治疗的小鼠和紧肤 1 小鼠中,Tamibarotene (Am80)/他米巴罗汀(1 mg/kg/天)分别显着减少真皮和皮下纤维化。他米巴罗汀持续显着抑制 BLM 治疗小鼠病变皮肤中与组织纤维化相关的几种分子的表达,包括转化生长因子-β1、结缔组织生长因子、IL-4、IL-10、IL-13、IL- 17A,肿瘤坏死因子-α、IFN-γ和单核细胞趋化蛋白1。此外,在用BLM治疗的小鼠的引流淋巴结中,他米巴罗汀增加了幼稚T细胞的比例,并降低了CD4+ T中效应T细胞的比例细胞[2]。与未接受治疗的小鼠相比,患有牙周炎的小鼠在接受他米巴罗汀(2.5 mg/kg,口服)后血清中 AST、ALT 或 ALP 水平没有表现出任何明显的变化。他米巴罗汀可减少牙龈卟啉单胞菌诱导的小鼠破骨细胞的数量并增强牙槽骨吸收。与 EPD 小鼠相比,他米巴罗汀显着提高了 CD4+ Foxp3+ Treg 细胞的百分比。此外,他米巴罗汀成功降低了 P 中 Th17 (CD4+ROR-γt+) 细胞的表达。CLN 和牙龈组织被牙龈线虫感染[3]。当给予他米巴罗汀(1 mg/kg,口服)时,动脉球囊损伤的大鼠表达更多 apelin,这与培养的 VSMC 的研究结果一致 [4]。服用他米巴罗汀(1 mg/kg/天)后,老年 SAMP8 小鼠的海马 ADAM10 水平有所改善。服用他米巴罗汀后,Hes5和Ki67恢复,空间工作记忆增强[5]。
他米巴罗汀(Am80)是一种合成维甲酸,可调节各种自身免疫性和炎症性疾病及其动物模型的病理过程。我们在这里使用动物模型研究了他米巴罗汀(Am80)对系统性硬化症的治疗潜力。Am80分别显著减轻了博莱霉素(BLM)治疗的小鼠和皮肤紧绷1小鼠的皮肤和皮下纤维化。一致地,他米巴罗汀(Am80)显著抑制了BLM治疗小鼠皮损皮肤中与组织纤维化相关的各种分子的表达,包括转化生长因子β1、结缔组织生长因子、IL-4、IL-10、IL-13、IL-17A、肿瘤坏死因子-α、IFN-γ和单核细胞趋化蛋白1。此外,Am80降低了BLM治疗小鼠引流淋巴结中CD4+T细胞中效应T细胞的比例,同时增加了幼稚T细胞的数量。[2] 他米巴罗汀(Am80)是一种合成的视黄酸受体(RAR),是一种对RARα和RARβ具有高度特异性的激动剂。维甲酸激动剂已被证明可以抑制Th17细胞极化,并在炎症性疾病过程中增强叉头盒P3(Foxp3)的表达。本研究的目的是评估Am80在口腔微环境中调节牙周炎免疫反应方面以前未被认识的作用。通过口腔感染牙龈卟啉单胞菌W83诱导小鼠牙周炎实验模型。我们的结果表明,Am80有效地抑制了牙龈卟啉单胞菌W83诱导的牙槽骨吸收,并减少了破骨细胞的数量。我们阐明,这些影响与牙龈组织、颈部淋巴结和脾脏中CD4(+)类维生素A相关孤儿受体(ROR)γt(+)细胞百分比的降低和CD4(+。此外,在牙龈卟啉单胞菌感染的小鼠中,Am80下调了白细胞介素-17A(IL-17A)、核因子κβ配体受体激活剂(RANKL)、单核细胞趋化蛋白-1(MCP-1)、IL-6和IL-1β的mRNA表达水平。同时,Am80上调了牙龈组织和CLN中IL-10和转化生长因子β1(TGF-β1)的表达水平。我们的研究结果表明,Am80可以防止牙周骨吸收,主要是通过调节口腔微环境中的免疫反应,并证明了Am80作为预防牙周炎的新临床策略的潜力。[3] 一项体内研究表明,他米巴罗汀(Am80)增加了大鼠球囊损伤动脉中apelin的表达,这与培养的VSMCs的结果一致。[4] 视黄酸(RA,一种维生素a代谢产物)受体(RAR)是一种转录因子。维生素A/RA给药可改善小鼠模型中阿尔茨海默病(AD)和与年龄相关的记忆/学习衰退。最近,一种含去整合素和金属蛋白酶结构域的蛋白质10(ADAM10)被鉴定为RA介导的抗AD机制中的关键分子。我们研究了长期服用RAR激动剂Tamibarotene(Am80)对衰老加速小鼠(SAMP8)ADAM10表达的影响。此外,我们估计了ADAM10介导的淀粉样前体蛋白(APP)、淀粉样β蛋白(Aβ)和分裂毛/增强子(Hes)表达的变化。在这些小鼠中还评估了空间工作记忆和海马增殖标志物(Ki67)的水平。13个月龄SAMP8小鼠海马ADAM10 mRNA和蛋白表达显著降低;Am80给药后,它们的表达显著改善。此外,Am80给药后,Hes5和Ki67的表达水平恢复,工作记忆的恶化得到抑制,而APP和Aβ水平保持不变。我们的研究结果表明,Am80给药通过激活海马ADAM10Notch-Hes5增殖通路有效改善了痴呆症[5]。 |
| 酶活实验 |
siRNA转染[4]
Sigma-Aldrich设计合成了针对大鼠RARα和KLF5序列的siRNA,Invitrogen设计合成了对抗大鼠apelin序列的siRNA。使用si-NS(非特异性siRNA)和Sp1特异性siRNA。针对RARα的siRNA序列(si-RARα)为5′-CUCACAACGUGUCUCU-3′和5′-AGACACGUGUGUCUG-3′。针对apelin(si-apelin)的siRNA序列为5′-GGCUAGAGAGCAACAUTT-3′和5′-AUGUAGUCUCUCUCUACCTT-3′。按照制造商的说明,使用Lipofectamine™试剂进行输血。转染后24小时,用或不用他米巴罗汀(Am80)(4μM)处理VSMCs。然后收获细胞并用于PCR、qRT-PCR和蛋白质印迹分析。 EMSA[4] 根据制造商的建议,使用LightShift化学发光EMSA试剂盒进行EMSA。简而言之,合成了一种含有apelin启动子TCE-1位点的生物素标记探针。序列如下:5′-CTCCTGCCTCCCCCCCCCCCCCCCCTCTCTCTCTTCTAATGACCAC-3′。将用Tamibarotene (Am80)处理24小时的VSMCs的总共5μg核蛋白与生物素标记的探针在室温下孵育30分钟,装载到6%的非变性聚丙烯酰胺凝胶上,在100 V下运行40分钟,然后电转移到尼龙膜上。通过暴露于254 nm紫外线辐射15分钟,将探针交联到膜上。最后,根据制造商的说明,通过化学发光检测生物素标记的探针。为了特异性鉴定结合复合物中的KLF5、RARα和Sp1蛋白,将2mg抗KLF5、抗RARα及抗Sp1抗体加入到结合反应混合物中,并在室温下孵育30分钟,然后加入探针。 |
| 细胞实验 |
萤光素酶报告检测[1]
RARE报告子和pCI空载体或pCI-RARA(野生型)通过电穿孔共转染到HuT78细胞中。恢复24小时后,用含有2%炭剥离FBS的RPMI中的他米巴罗汀(Am80)或载体(二甲亚砜)处理转染细胞24小时。如前所述,使用Centro XS3 LB 960微孔板光度计,按照制造商的协议,使用双荧光素酶报告分析系统测量荧光素酶表达。每种裂解物的总蛋白浓度用于归一化。 转录组分析[1] Mac-1、Karpas 299和HuT78细胞在含有2%炭剥离FBS的培养基中用他米巴罗汀(Am80)(0μM、20μM或40μM)、ATRA(0μM、10μM或20μM)或贝沙罗汀。如前所述进行RNA测序。简而言之,在提取总RNA后,构建mRNA文库,并在Illumina HiSeq仪器上进行100碱基对配对末端测序。测序数据被映射到hg19,并保留了至少一个样本中至少100个读数的基因进行分析。每种药物的剂量依赖性效应是通过配对t检验(EdgeR)进行中低和高中比较来确定的。如果基因在两次比较中都符合倍数变化和P值标准(log2倍数变化≥0.5或≤-0.5,P值≤0.05),则认为基因表达不同。GSEA是在0μM与40μM AM80比较的折叠变化符号和对数(P值)的乘积生成的排名数据集上进行的。使用Ingenuity Pathway analysis软件对差异表达的基因进行通路分析。 巨噬细胞极化[2] 如前所述,从野生型小鼠制备巯基乙酸诱导的腹腔巨噬细胞(Matsumoto等人,2007)。在存在或不存在他米巴罗汀(Am80)的情况下,用20ng/ml的IL-4刺激24小时,将培养过夜的腹膜巨噬细胞极化为M2巨噬细胞。从培养的细胞中分离总RNA,并通过定量实时逆转录PCR进行分析。此外,巨噬细胞用抗CD204和抗CD206抗体染色,并用流式细胞术定量。 THP-1细胞的细胞培养[2] THP-1细胞购自美国典型培养物保藏中心。处于对数生长期的THP-1细胞用100ng/ml的IFN-γ预处理6小时,并在有或没有他米巴罗汀(Am80)的情况下用100ng/ml脂多糖刺激24小时。如上所述,从细胞裂解物中分离出总RNA。 人皮肤微血管内皮细胞的细胞培养[2] 使用人皮肤微血管内皮细胞。在用0.5 ng/ml TNF-α刺激前1小时,用指定浓度的他米巴罗汀(Am80)或等量的DMSO处理人皮肤微血管内皮细胞。将细胞再培养24小时。如上所述,从细胞裂解物中分离总RNA。 免疫印迹[2] 在有或没有他米巴罗汀(Am80)的情况下,用TGF-β1刺激人皮肤成纤维细胞24小时。制备细胞裂解物并进行免疫印迹,如前所述(Noda等人,2014)。 细胞、细胞培养和处理[4] 雄性Sprague-Dawley大鼠(80-100g)用氯胺酮(60mg/kg体重)和甲苯噻嗪(5mg/kg体重)腹腔麻醉,并如前所述从胸主动脉提取VSMCs。接下来,在麻醉下通过放血杀死大鼠。VSMCs在37°C、5%CO2的加湿气氛中,在添加了10%FBS的DMEM(Dulbecco改良Eagle培养基)中维持。本研究中使用的细胞传代三到六代。在他米巴罗汀(Am80)刺激之前,VSMCs在无血清DMEM中维持24小时。然后将其在含有2%FBS和4μMTamibarotene(Am80)的DMEM中培养指定时间。 |
| 动物实验 |
Mice:For the infection, mice are given sulfamethoxazole and trimethoprim in an oral suspension at 10 mL of deionized water ad libitum for 10 days to reduce the native flora and to support colonization of P. gingivalis W83. Four days after the antibiotic therapy finishes, periodontal infection is established through oral inoculation using 1010 colony-forming units of P. gingivalis suspended in 100 μL 4% carboxymethyl cellulose (CMC) for 7 days. The mice are euthanized 4 weeks after the first oral inoculation. Tamibarotene (2.5 mg/kg) is suspended in a 0.5% carboxymethyl cellulose solution. The drug is orally gavaged into the esophagus daily in a volume of 0.1 mL/10 g body weight. Tamibarotene is administered 1 h before the induction of periodontitis and then given daily per the protocol until day 28. Control mice with periodontal disease receive the same volume of 0.5% carboxymethyl cellulose solution. The body weight of each mouse is measured every 3 days.
TSK1 mice [2] TSK1 mice were used. Genotyping of TSK1 mice was performed by PCR with the following primers (forward 5′-GTTGGCAACTATACCTGCAT-3′ and reverse 5′-CCTTTCCTGGTAACATAGGA-3′). Tamibarotene (Am80) treatment to TSK1 mice was began at the age of 3 weeks and finished at the age of 7 weeks. Tamibarotene (Am80) treatment [2] Feeding pellets were made by mixing Am80 with a commercial rodent diet. Approximately 1 mg/kg/day Am80 were administered to mice, according to the procedure of Miwako and Shudo (2009). Administration was started 1 week before BLM or PBS subcutaneous injection. Mouse model of periodontitis [3] Periodontal infection was induced by P. gingivalis W83 as described previously, with minor modifications. P. gingivalis W83 was cultivated on a brain–heart infusion (BHI) agar, supplemented with Vitamin K1 (10 μg/mL), hemin (0.25%), and sterile defibrinated sheep blood (5%). The bacteria were incubated in an anaerobic chamber at 37 °C for 7 days in a 10% CO2, 5% H2, and 85% N2 atmosphere. The bacteria were collected and cultured in complete-BHI liquid at 37 °C for 24 h and then used during the logarithmic growth phase for oral infection. For the infection, mice were given sulfamethoxazole and trimethoprim in an oral suspension at 10 mL of deionized water ad libitum for 10 days to reduce the native flora and to support colonization of P. gingivalis W83. Four days after the antibiotic therapy finished, periodontal infection was established through oral inoculation using 1010 colony-forming units of P. gingivalis suspended in 100 μL 4% carboxymethyl cellulose (CMC) for 7 days. The mice were euthanized 4 weeks after the first oral inoculation. Drug treatments [3] Tamibarotene (Am80) (2.5 mg/kg) was suspended in a 0.5% carboxymethyl cellulose solution. The drug was orally gavaged into the esophagus daily in a volume of 0.1 mL/10 g body weight. Am80 was administered 1 h before the induction of periodontitis and then given daily per the protocol until day 28. Control mice with periodontal disease received the same volume of 0.5% carboxymethyl cellulose solution. The body weight of each mouse was measured every 3 days. Balloon injury model [4] Male Sprague–Dawley rats were anaesthetized with ketamine (60 mg/kg of body weight) and xylazine (5 mg/kg of body weight) intraperitoneally. Briefly, after a median incision on the anterior neck, the carotid arteries of the left side were isolated and a distal incision was made. After the blood was removed, a 0.13-mm-diameter balloon catheter was advanced gently into the left common carotid artery. The balloon was inflated with saline to distend the common carotid artery and then pulled back to the external carotid artery. The catheter was withdrawn and the proximal end of the external carotid artery was ligated, and blood flow was re-established after removing the clamps on the arteries. All procedures were performed by a single operator. Tamibarotene (Am80) was administrated orally at a dose of 1 mg/kg of body weight per day beginning 1 day before balloon injuring and continuing for 14 days thereafter. On day 14 after injury and administration of Am80 orally, the rats were killed by exsanguination under anaesthesia, and the carotid artery was collected for qRT-PCR or immunohistochemistry. The animals used were male SAMP8 and SAMR1 mice (Japan SLC, Shizuoka, Japan) aged 2–13 months at the beginning of the experiment. The animals were maintained on a 12:12 h light:dark cycle (lights on at 09:00) at an ambient temperature of 23 ± 1 °C and had free access to food and water. An Tamibarotene (Am80)-containing diet was prepared to a concentration of 0.001% (w/w) by mixing the drug with the control diet (MF, 360 kcal/100 g; Oriental Yeast, Tokyo, Japan). The concentration of Tamibarotene (Am80) was equivalent to 1 mg/kg/day for a mouse weight of 40 g and a food consumption of 4 g/day. The Am80-containing and control diets were fed for 4 weeks to each group before radial maze test setting. Obvious adverse effects were not observed in the Am80-treated group.[5] |
| 毒性/毒理 (Toxicokinetics/TK) |
Protein Binding
Over 99%, predominantly to serum albumin. |
| 参考文献 |
|
| 其他信息 |
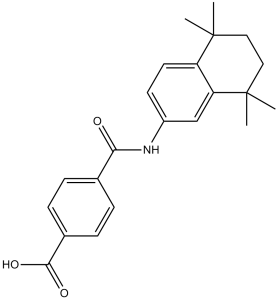
Tamibarotene is a dicarboxylic acid monoamide resulting from the condensation of one of the carboxy groups of terephthalic acid with the amino group of 5,5,8,8-tetramethyl-5,6,7,8-tetrahydronaphthalen-2-amine. It has a role as an antineoplastic agent and a retinoic acid receptor alpha/beta agonist. It is a member of tetralins, a retinoid and a dicarboxylic acid monoamide. It is functionally related to a 4-carbamoylbenzoic acid and a terephthalic acid.
Tamibarotene is a novel synthetic retinoid for acute promyelocytic leukaemia (APL). Tamibarotene is currently approved in Japan for treatment of recurrent APL, and is undergoing clinical trials in the United States. Tamibarotene is an orally active, synthetic retinoid, developed to overcome all-trans retinoic acid (ATRA) resistance, with potential antineoplastic activity. As a specific retinoic acid receptor (RAR) alpha/beta agonist, tamibarotene is approximately ten times more potent than ATRA in inducing cell differentiation and apoptosis in HL-60 (human promyelocytic leukemia) cell lines in vitro. Due to a lower affinity for cellular retinoic acid binding protein (CRABP), tamibarotene may show sustained plasma levels compared to ATRA. In addition, this agent may exhibit a lower toxicity profile than ATRA, in part, due to the lack of affinity for the RAR-gamma receptor, the major retinoic acid receptor in the dermal epithelium. Drug Indication Investigated for use/treatment in leukemia (unspecified). Mechanism of Action Tamibarotene is a specific agonist for retinoic acid receptor alpha/beta with possible binding to retinoid X receptors (RXR). Pharmacodynamics Tamibarotene is a new synthetic retinoid drug recently approved for relapsed or refractory acute promyelocytic leukemia (APL) in Japan. It is a specific agonist for retinoic acid receptor alpha/beta. Compared to all-trans retinoic acid (ATRA), a natural retinoid indicated for a first-line treatment of APL, tamibarotene is chemically more stable and several times more potent as an inducer of differentiation in promyelocytic leukemia cells. In contrast to ATRA, whose plasma concentration declines considerably during daily administration, tamibarotene sustains plasma level probably due to a lower affinity for cellular retinoic acid binding protein. Furthermore, adverse side effects were milder than those of ATRA in clinical trials. Peripheral T-cell lymphomas (PTCLs) are aggressive non-Hodgkin lymphomas with generally poor outcomes following standard therapy. Few candidate therapeutic targets have been identified to date. Retinoic acid receptor alpha (RARA) is a transcription factor that modulates cell growth and differentiation in response to retinoids. While retinoids have been used to treat some cutaneous T-cell lymphomas (CTCLs), their mechanism of action and the role of RARA in CTCL and other mature T-cell lymphomas remain poorly understood. After identifying a PTCL with a RARAR394Q mutation, we sought to characterize the role of RARA in T-cell lymphoma cells. Overexpressing wild-type RARA or RARAR394Q significantly increased cell growth in RARAlow cell lines, while RARA knockdown induced G1 arrest and decreased expression of cyclin-dependent kinases CDK2/4/6 in RARAhigh cells. The retinoids, AM80 (tamibarotene) and all-trans retinoic acid, caused dose-dependent growth inhibition, G1 arrest, and CDK2/4/6 down-regulation. Genes down-regulated in transcriptome data were enriched for cell cycle and G1-S transition. Finally, RARA overexpression augmented chemosensitivity to retinoids. In conclusion, RARA drives cyclin-dependent kinase expression, G1-S transition, and cell growth in T-cell lymphoma. Synthetic retinoids inhibit these functions in a dose-dependent fashion and are most effective in cells with high RARA expression, indicating RARA may represent a therapeutic target in some PTCLs. [1] |
| 分子式 |
C22H25NO3
|
|
|---|---|---|
| 分子量 |
351.44
|
|
| 精确质量 |
351.183
|
|
| 元素分析 |
C, 75.19; H, 7.17; N, 3.99; O, 13.66
|
|
| CAS号 |
94497-51-5
|
|
| 相关CAS号 |
|
|
| PubChem CID |
108143
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 密度 |
1.2±0.1 g/cm3
|
|
| 沸点 |
449.6±45.0 °C at 760 mmHg
|
|
| 熔点 |
231-232ºC
|
|
| 闪点 |
225.7±28.7 °C
|
|
| 蒸汽压 |
0.0±1.2 mmHg at 25°C
|
|
| 折射率 |
1.593
|
|
| LogP |
6.48
|
|
| tPSA |
66.4
|
|
| 氢键供体(HBD)数目 |
2
|
|
| 氢键受体(HBA)数目 |
3
|
|
| 可旋转键数目(RBC) |
3
|
|
| 重原子数目 |
26
|
|
| 分子复杂度/Complexity |
546
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
O=C(O)C1=CC=C(C(NC2=CC=C3C(C)(C)CCC(C)(C)C3=C2)=O)C=C1
|
|
| InChi Key |
MUTNCGKQJGXKEM-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C22H25NO3/c1-21(2)11-12-22(3,4)18-13-16(9-10-17(18)21)23-19(24)14-5-7-15(8-6-14)20(25)26/h5-10,13H,11-12H2,1-4H3,(H,23,24)(H,25,26)
|
|
| 化学名 |
4-[(5,5,8,8-tetramethyl-6,7-dihydronaphthalen-2-yl)carbamoyl]benzoic acid
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (7.11 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (7.11 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (7.11 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.8454 mL | 14.2272 mL | 28.4544 mL | |
| 5 mM | 0.5691 mL | 2.8454 mL | 5.6909 mL | |
| 10 mM | 0.2845 mL | 1.4227 mL | 2.8454 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT06289998 | Recruiting | Drug: Tamibarotene Drug: Placebo |
Autosomal Dominant Polycystic Kidney Disease (ADPKD) |
Rege Nephro Co., Ltd. | December 22, 2023 | Phase 2 |
| NCT06085638 | Withdrawn | Drug: Tamibarotene Drug: Venetoclax |
Chronic Myelomonocytic Leukemia | M.D. Anderson Cancer Center | August 23, 2017 | Phase 1 Phase 2 |
| NCT04905407 | Recruiting | Drug: Tamibarotene Drug: Venetoclax |
Acute Myeloid Leukemia | Syros Pharmaceuticals | August 26, 2021 | Phase 2 |
| NCT01337154 | Terminated | Drug: Tamibarotene Drug: Placebo |
Stage IIIB Non-small Cell Lung Cancer With Pleural Effusion |
CytRx | April 2011 | Phase 2 |
|
|---|
|
|